FDA approves first vaccine for RSV after decades of attempts
WASHINGTON — The U.S. approved the first vaccine for RSV on Wednesday, allowing the sale of shots to protect older adults from a respiratory virus that’s most notorious for attacking babies but endangers their grandparents too.
The Food and Drug Administration decision makes GSK’s shot, called Arexvy, the first of several potential RSV vaccines in the pipeline to be licensed anywhere.
The move sets the stage for adults 60 and older to get vaccinated this fall — but first, the U.S. Centers for Disease Control and Prevention must decide whether every senior needs the protection from respiratory syncytial virus, or only those considered at high risk from RSV. The CDC’s advisors will debate that question in June.
After decades of failure in the quest for an RSV vaccine, doctors are eager to finally have something to offer their patients for protection — especially after a virus surge that strained hospitals last fall.
Children’s hospitals in L.A., Orange County, the Bay Area and elsewhere in the state are being stressed by respiratory syncytial virus, or RSV.
“This is a great first step ... to protect older persons from serious RSV disease,” said Dr. William Schaffner, medical director of the National Foundation for Infectious Diseases and an infectious disease expert at Vanderbilt University, who wasn’t involved in the vaccine’s development.
“We’re going to be working our way down the age ladder” for what’s expected to be a string of new protections, he said.
The FDA is considering competitor Pfizer’s similar vaccine for older adults. Pfizer also is seeking approval to vaccinate pregnant women so that babies are born with some protection.
There isn’t a vaccine for children, but high-risk infants often get monthly doses of a protective drug during RSV season — and European regulators recently approved the first one-dose option.
The FDA also is considering whether to approve one-shot medicines by Sanofi and AstraZeneca.
“This is a very exciting time with multiple potential RSV solutions coming out after years of really nothing,” said Dr. Phil Dormitzer, chief of vaccine research and development for GSK.
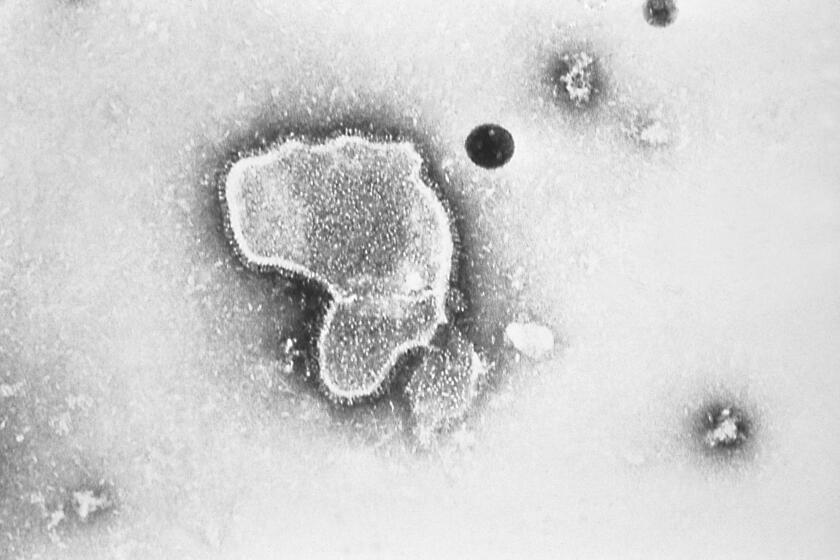
RSV is a cold-like nuisance for most people, but it can be life-threatening for the very young, the elderly and people with certain health problems. It can impede babies’ breathing by inflaming their tiny airways, or creep deep into seniors’ lungs to cause pneumonia.
In the U.S., about 58,000 children under 5 are hospitalized for RSV each year, and several hundred die. Among older adults, as many as 177,000 are hospitalized with RSV and up to 14,000 die annually.
With Orange County declaring a health emergency due to high RSV cases, concern about the virus is peaking. Here’s what you should know about it.
It’s taken longer than usual to come up with this vaccine. Efforts suffered a major setback in the 1960s when an experimental shot worsened infections in children. Scientists finally figured out a better way to develop such vaccines — but modern candidates have still been tested with adults first.
GSK’s new vaccine for older adults trains the immune system to recognize a protein on RSV’s surface, and contains an ingredient called an adjuvant to further rev up the immune reaction.
In an international study of about 25,000 people 60 and older, one dose of the vaccine was nearly 83% effective at preventing RSV lung infections, and reduced the risk of severe infections by 94%.
To see how long protection lasts, GSK is tracking study participants for three years, comparing those who get just one vaccination in that period with others who are given yearly boosters.
Reactions to the vaccinations have been typical, such as muscle pain and fatigue.
A new study suggests vaccinating pregnant women protects their newborns from the common but scary respiratory virus called RSV.
There have been hints of rare but serious risks among study participants — one case of Guillain-Barre syndrome, which can cause usually temporary paralysis, and two cases of a type of brain and spinal cord inflammation. The FDA is requiring the company to continue studying whether there is a link to the vaccine.
If the CDC ultimately recommends the vaccination for some or even all seniors, it will mean another shot in the fall along with their yearly flu vaccines — and possible COVID-19 boosters.
“We’ll have to educate the population,” Schaffner said, “that this virus that not everyone has heard about is actually an important threat to their health in the wintertime.”