Snooping around in hospital pipes, scientists find DNA that fuels the spread of superbugs
The pipes carrying away the effluvia of very sick people are bound to be nasty, dirty places.
But just how unwholesome they are is made clear in a new report showing that the pipes beneath a hospital intensive care unit are a throbbing, seething hookup zone for antibiotic-resistant bacteria.
Even when the sinks, faucets, bedrails and countertops of patients’ rooms are largely free of germs that resist modern medicines, the genetic building blocks for antibiotic resistance intermingle freely in the pipes connected to those rooms, according to a study published Tuesday in the journal mBio. That DNA can give superbugs the power to defeat modern medicines and threaten the lives of patients.
In 2012, a team of sleuths at the National Institutes of Health’s Clinical Care Center broke new ground in the effort to rout a deadly antibiotic-resistant infection that sickened at least 17 patients — and killed six — during the summer of 2011. Using whole-genome sequencing for the first time in such an investigation, the team identified the culprit: a super-potent strain of Klebsiella pneumoniae bacteria carried into the center by a seriously ill woman from New York.
The microbe, they discovered, was probably traveling between rooms after lodging in sinks and drains. Some were ripped out and others were sanitized in an extensive eradication effort.
The new study involves some of the same NIH investigators. Over five years, they used ever-improving genome sequencing techniques to hunt down and track antibiotic-resistant germs.
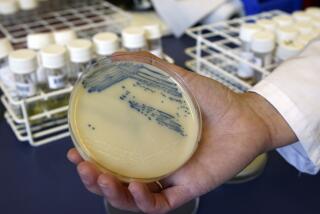
In all, they gathered and sequenced the genomes of 700 different samples from the same NIH hospital, looking specifically for carbapenem-resistant Enterobacteriaceae.
The problem of antibiotic resistance is a growing worldwide threat. The Centers for Disease Control and Prevention estimate that each year, more than 2 million patients in the U.S. are infected with a bacterium that’s become resistant to one or more antibiotic medications, and that at least 23,000 of them die as a result.
Carbapenems are antibiotic drugs of “last resort” that are given to hospital patients who develop infections from pathogens that have defeated several other medications.
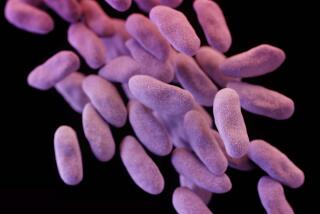
Carbapenem-resistant Enterobacteriaceae — including strains of the commonplace gut bacteria Klebsiella and Escherichia coli (E. coli) — most often infect hospital and nursing-home patients who take long courses of antibiotic medicines, and whose care requires devices like ventilators, urinary catheters or intravenous lines.
And they’re deadly: One report suggests that they contribute to death in up to 50% of patients who become infected.
In addition to swabbing patients and testing the usual suspects — sinks, computers, doorknobs, counters — the team collected samples from pipes beneath the ICU of the NIH’s Clinical Care Center, and from outside manholes draining hospital wastewater.
Then they conducted whole-genome analyses on the samples to study the bacterial plasmids, the readily transferable rings of DNA that can confer antibiotic resistance from one bacterium to another.
In samples taken from places patients and their caregivers touched most, the results were largely reassuring — only 3 of 217 (1.4%) tested positive for the antibiotic-resistant organisms that most threaten patients.
In addition, out of 340 samples collected from drains, only 11 (3.2%) were positive.
When those samples were compared with others taken from patients, they were not the same. In short, the infection-control efforts of one of the nation’s most elite hospitals seemed to be working pretty well.
But the story was quite different for the samples from pipes and sewers — all of the samples drawn from the piping system leading from the ICU tested positive for bacterial plasmids that confer resistance to carbapenems. And so did all seven samples drawn from wastewater samples taken from two external manholes associated with the NIH Clinical Center.
Two wastewater samples collected from a non-ICU floor of the hospital came up negative for carbepanem-resistant bacteria.
Scientists long believed that antibiotic resistance develops mainly when a bacterium comes into repeated contact with an antibiotic — say, in a patient’s intestinal tract — and eventually evolves a defense against it. Lax personal hygiene or hospital cleaning practices could then scatter those fortified germs to places where they could infect additional patients and spread their antibiotic-resistant superpowers.
That’s why efforts to curb the growing problem of antibiotic resistance have focused both on reducing the unnecessary use of antibiotic medicines and, in hospitals, deploying armies of workers to locate and shut down germy breeding grounds.
But far from view, in the hospital’s fecund wastewater pipes, a bacterium that can still be vanquished with available antibiotics need only meet up with another that’s antibiotic-resistant. In the resulting exchange, the first bacterium acquires a plasmid from the second that will make it impervious to workhorse medicines.
That lateral gene transfer means bacteria can gain resistance without even being exposed to an antibiotic medicine. And, the study authors said, it’s probably happening just inches from where even the most vigilant hospital infection-control teams are taking samples and directing cleaning crews to scrub: on the other side of sink drains, of housekeeping floor drains and in the pipes leading from toilets.
One of the study’s lead authors, NIH microbiologist Karen Frank, said the new investigation probably uncovers a dynamic at work inside and beneath most hospitals in the United States. Hospital infection-control teams need to focus on those wet, unseen quarters to ensure their contents are sequestered from patients.
Does a hospital’s outflow threaten users of public drinking water?
Frank said the prevalence of antibiotic-resistant bacterial infection has increased in the U.S. over the last three decades. But “there has been no evidence to suggest that antibiotic-resistant bacteria in wastewater from hospitals, households, or other sources are contaminating drinking water in the United States.”
Safety testing for public drinking water seems to be working.
But, she added, “the United States has better sewage treatment facilities than some areas of the world with more resistant bacteria and fewer resources.”
The spread of resistant bacteria — an escalating problem that recognizes no national boundaries — “should be addressed in all countries,” she said.
MORE IN SCIENCE
Humanity will need to make some drastic changes if it wants to keep the ‘good life’ going
Radiation from cellphones is not hazardous to your health, government scientists say
Why diets backfire: A year or more after weight loss, the desire to eat grows stronger
UPDATES:
5:10 p..m.: This report has been updated with additional details.
This was originally published at 2:20 p.m.