Antibiotics wreak havoc on the gut. Can we kill the bad bugs and spare the good ones?
Inside every human is a thriving zoo of bacteria, fungi, viruses and other microscopic organisms collectively known as the microbiome. Trillions of microbes live in the digestive tract alone, a menagerie estimated to contain more than 1,000 species.
This ecosystem of tiny stuff affects our health in ways science is only beginning to understand, facilitating digestion, metabolism, the immune response and more. But when serious infection sets in, the most powerful antibiotics take a merciless approach, wiping out colonies of beneficial bacteria in the digestive tract and often prompting secondary health problems.
“Increasingly, researchers are recognizing the benefits of protecting the human gut microbiome, particularly because its integrity and diversity is linked to metabolic influences on mental health and physical health conditions,” said Dr. Oladele A. Ogunseitan, a professor of population health and disease prevention at UC Irvine.
Drug-resistant bugs are evolving faster than new medicines are being developed, rendering the current arsenal of medicines increasingly ineffective. But the more we understand about the microbiome, the clearer it is that we need antibiotics that are discerning in their targets.
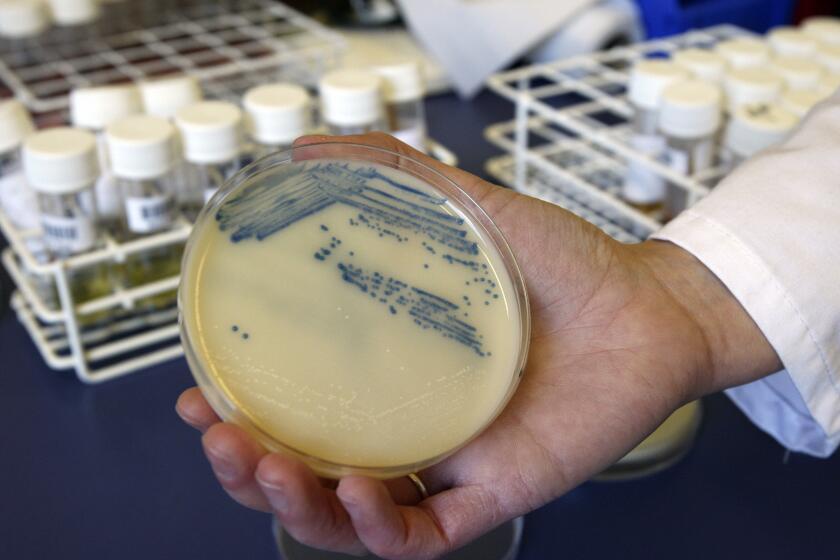
With that goal in mind, a chemistry team at the University of Illinois Urbana-Champaign is experimenting with a compound that attempts to address both problems. The antibiotic, lolamicin, both successfully vanquished several drug-resistant pathogens in mice while sparing the animals’ microbiome. The results were published in the journal Nature.
“Only recently has it been recognized that killing these [beneficial] bacteria is having many deleterious effects on patients,” said Paul J. Hergenrother, a chemistry professor at the University of Illinois Urbana-Champaign who co-led the study. “We have been interested for some time in finding antibiotics that would be effective without killing the good bacteria.”
Diane Shader Smith’s daughter, Mallory Smith, died at age 25 after fighting an antibiotic-resistant lung infection for 12 years. A new book of her daughter’s diary entries and a website are aimed at finding solutions.
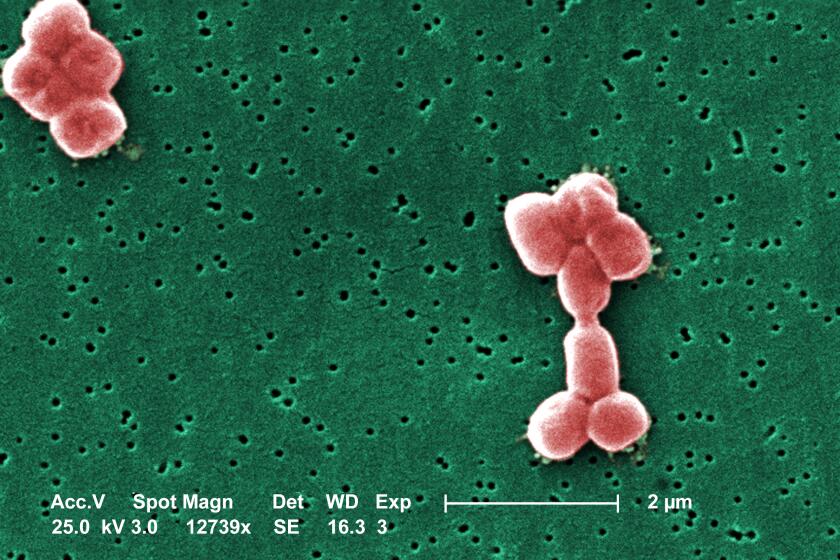
The team set out to create an antibiotic that would both preserve the gut microbiome while targeting gram-negative bacteria, a particularly hardy category of superbugs. Encased in both an inner and outer membrane that antibiotics struggle to cross, gram-negative bacteria are resistant to most currently available therapies. They are responsible for the majority of the estimated 35,000 deaths in the U.S. each year from drug-resistant infections, according to the U.S. Centers for Disease Control and Prevention.
Worldwide, antimicrobial resistance kills an estimated 1.27 million people directly every year and contributes to the deaths of millions more.
Not all gram-negative bugs make us sick. Bacteria populations in the average human gut are roughly split between gram-negative and gram-positive types, said Kristen Munoz, a former doctoral student at the University of Illinois who co-led the study.
Broad spectrum antibiotics can’t tell which bugs to spare, she said. As a result, anything strong enough to treat a bad infection “is going to wipe out a good amount of your gut microbiome,” she said, even though they “aren’t doing anything wrong.”
The team focused its search for a new drug on compounds that suppress the Lol system, which shuttles lipoproteins between the inner and outer membranes in gram-negative bacteria.
The Lol system’s genetic code looks different in harmful bacteria than it does in beneficial ones, which suggested to researchers that medicines that targeted the Lol system would be able to distinguish good bugs from bad ones.
The team designed multiple versions of these Lol-inhibiting compounds. When tested against 130 drug-resistant strains of Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae, one in particular proved especially potent.
They tested this antibiotic, which they named lolamicin, on mice that had been infected with drug-resistant strains of septicemia or pneumonia. All of the mice with septicemia survived after receiving lolamicin, as did 70% of the mice with pneumonia.
To measure the effect on gut bacteria, the researchers gave healthy mice either lolamicin, a placebo or one of two common antibiotics, amoxicillin and clindamycin. After collecting baseline stool samples, they sampled the animals’ poop seven, 10 and 31 days after treatment.
Mice treated with amoxicillin or clindamycin had lower beneficial bacteria counts and less diversity of gut bacteria. In contrast, the guts of lolamicin-treated mice appeared largely the same.
“It was exciting to see that lolamicin did not really cause any changes in the microbiome, whereas the other clinically used antibiotics did,” Munoz said.
Scientists have created a synthetic molecule that appears capable of attacking a broad range of bacteria that have become dangerously resistant to workhorse medicines.
A disrupted microbiome can have immediate consequences for people battling infection. When beneficial microbes are decimated, dangerous bugs have fewer competitors and secondary infections can take hold.
Clostridium difficile is a notorious opportunistic pathogen, so the researchers did an experiment where they exposed mice treated with lolamicin, amoxicillin or clindamycin to C. difficile. The mice who took standard antibiotics were soon crawling with C. difficile. The lolamicin mice showed little to no infection.
The lab hopes to one day take lolamicin or a version of it to clinical trials, Hergenrother said. (Munoz received her doctorate last year and now works as a scientific analyst in Los Angeles.) Yet these are still early days for the drug. While the concept of a discerning antibiotic is a welcome development, it must clear significant barriers before it could make a difference for patients.
“Distinguishing a quote-unquote ‘bad bug’ from a quote-unquote ‘good bug’ is not always as straightforward as it may seem,” said Dr. Sean Spencer, a Stanford University gastroenterologist and physician scientist who was not involved with the research.
Some beneficial bugs in the gut bear a striking genetic resemblance to harmful pathogens, he said. Others are benign in some contexts and dangerous in others: “In a critically ill individual, a good bug can do bad things.”
Years can pass between a new antibiotic’s proof of concept and its entry to the market, and the vast majority never make it to the end of that pipeline. It’s also not clear how easily or how quickly bacteria will develop resistance, which is perhaps the most formidable obstacle that lolamicin or any new antibiotic faces.
“One of the biggest problems is that bacteria are so smart. You can tackle one particular protein system or protein target in bacteria, but they will quickly find a resistance mechanism,” Munoz said. “They just have so many inherent mechanisms to overcome antibiotics.”
For the first time in half a century, researchers have identified a new antibiotic that appears to kill a deadly, drug-resistant superbug.