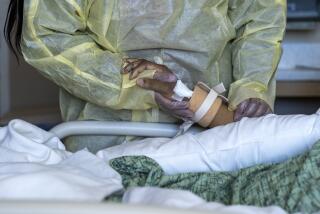
Explaining ECMO and how it saves lives
Have we ever been more aware of what it means to breathe? To fill our lungs with air, feel our chests expand and know that something so ordinary and commonplace is now — especially now — nothing to take for granted?
I would call the breath mystical if it weren’t so well understood. But understanding the principle of diffusion, by which oxygen and carbon dioxide change places in our bloodstream, does not make it any less extraordinary.
Aerobic function — every 3.3 seconds, an inhale, an exhale — is central to our species and occurred so early in our evolution that it is regulated in the brain stem in accordance with any physical activity, whether sitting at a computer keyboard or running in a marathon. With exquisite precision, neurons coordinate heart rate and breathing, constantly balancing the ever-changing chemistry of a body in motion.
When I first heard of a machine that performed this function and kept patients, especially COVID-19 patients, alive for weeks, I had to learn more. It goes by the name ECMO — for the process, extra-corporeal membrane oxygenation — and it seemed like something out of science fiction: It was either the height of medical arrogance or truly inspired.
An answer became clear once I got to know Blanca Lopez.
Her fate seemed a foregone conclusion. Lopez was infected with the novel coronavirus, and as a Latina between the ages of 30 and 49, belonged
to the region’s most vulnerable demographic.
“Research on animals was easy. What was bold was to hook it up to a patient.”
— Dr. Robert Bartlett, a developer of the ECMO technology
Yet ECMO saved her life, as it has saved thousands since it was first used in 1971 on a young man who had been in a car accident that had damaged his lungs. He was connected to the machine at Cottage Hospital in Santa Barbara for 36 hours and survived.
At the time, a young doctor who had helped developed ECMO, Dr. Robert Bartlett, was on the faculty at the newly opened medical school at UC Irvine. He had come west after a residency in Boston, where his mentor, cardiac surgeon Robert Gross, presented him with a challenge.
In the late 1950s, Gross was using a heart-lung bypass machine in the operating room, but he had encountered a problem. If surgery lasted more than an hour, white and red blood cells and platelets became damaged through contact with the hard surfaces of the device and to the air. Gross asked Bartlett to find a solution: a machine with no limits on its operational time.
Working in a research laboratory in the basement of Harvard’s medical school, Bartlett and an engineer from MIT, Phil Drinker, developed an airtight box that replicated a lung. Prototypes used a membrane of silicone rubber that allowed for the transfer of oxygen and carbon dioxide and protected the blood.
For the sickest of the COVID-19 sick, ECMO has become a final option.
The first candidates were dogs taken from the city pound. Bartlett emailed me a photograph showing Drinker feeding one of these animals as it was connected to the machine. Drinker looked rather nonchalant.
“When this finally worked,” I had to ask Bartlett, “did you feel you were standing next to God?”
Bartlett indulged me. “We’re all in the presence of God,” he said, adding, “research on animals was easy. What was bold was to hook it up to a patient.”
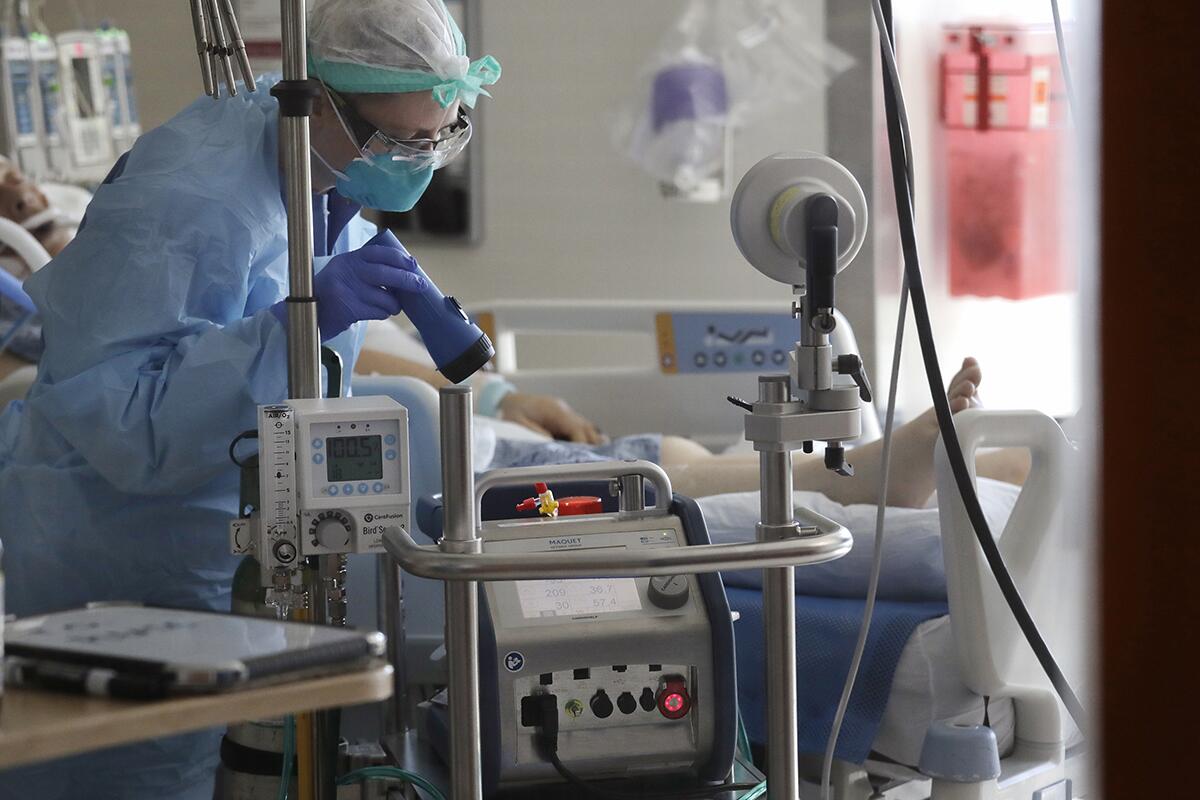
Following its successful use in Santa Barbara, more hospitals around the country started using ECMO, and researchers at the National Institute of Health enlisted it in a randomized clinical trial among adults at nine hospitals.
The outcomes were dreadful. Only 10% of the patients survived.
ECMO patient Blanca Lopez survived 51 days in the ICU. She finally got the chance to thank her medical team in person.
Bartlett, now a professor emeritus of surgery at the University of Michigan Medical School, cites flawed methodology of the trial, but by then, ECMO was considered a fringe technology.
But advancements in medicine and in medical devices are commonly marked by setbacks, and with the H1N1 pandemic, 30 years later, ECMO’s status changed.
The machine’s use had been mostly limited to children with heart defects and underdeveloped lungs and to adults awaiting organ transplants, but now it became a valuable tool for fighting the most severe symptoms of the flu, pneumonia and viral infections.
Vadim Gudzenko, an intensivist at Ronald Reagan UCLA Medical Center, became familiar with ECMO during that pandemic.
“The first time I saw ECMO’s effect on a patient, it was frankly mind-blowing,” he said. “I’ve been doing this for 10 years now, and it is amazing to see how people who were near death a minute ago are now alive and stable after the initiation of ECMO.”
From 2009 to 2019, the number of patients connected to the machine jumped from 3,262 to 15,875 per year. Close to 700 facilities around the world now offer ECMO.
But not everyone was on board. For decades, the FDA had approved the use of ECMO for only six hours, even though hospitals around the world employ the technology for as long as necessary for patient survival. (Last month, the federal agency granted one manufacturer long-term authorization.)
Bioethicists also question its value. ECMO is expensive, requires dedicated staffing and often leads to long stays in the intensive care unit. And its mortality rate is currently 49%, according to the Extracorporeal Life Support Organization, which monitors and studies the worldwide use of ECMO.
Some brand it as a “bridge to nowhere,” equipment that sustains the life of a patient who has no hope of recovery. As one doctor told Kaiser Health News in 2019, ECMO is “a fantastic example of ‘just because you can, doesn’t mean you should.’”
But the pandemic might be shifting this understanding. With no cure for COVID-19, physicians rely upon the body’s immune response to fight the infection. The challenge is to keep patients alive long enough for that battle to be waged successfully.
For most critically ill patients, a ventilator is sufficient, but for those with overwhelming lung damage, ECMO can be a better alternative. Still, Gudzenko is sensitive to the criticism that ECMO is risky.
“Because ECMO is an ultimate life support system, not all patients will make it,” he said, and the toll is hard not just on patients and their families but also on staff who commit themselves to saving these patients.
To ensure favorable outcomes, prospective ECMO patients are screened. They are typically younger than most hospitalized COVID-19 patients. They have no significant organ damage other than their lungs, and they have been on a ventilator for only a few days.
But a successful outcome does not always lead to a complete or immediate recovery. COVID-19 has also proven to be such a pernicious disease that patients, even if they survive, can experience lingering health problems.
Lopez’s story, however, gives Gudzenko hope. More than 22,000 people have died of COVID-19 in Los Angeles County. Many thousands more are grieving, trying to reconcile their losses with the demands of putting food on the table, struggling to hold onto their home, having to navigate a world where stepping outside brings new peril.
By saving Lopez’s life, her medical team saved the life of her family. Without ECMO, her elderly parents and her three children with disabilities, all of whom depend on her, would be in freefall.
There is no calculating the value of one life when so many lives are interconnected.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.