Millions of COVID-19 shots ordered for kids under 5 in anticipation, White House says
NEW YORK — Millions of COVID-19 vaccine doses have been ordered for very young children in anticipation of possible federal authorization of the shots next week, White House officials say.
The government allowed pharmacies and states to start placing orders last week, with 5 million doses initially available — half of them made by Pfizer and the other half made by Moderna, senior administration officials said.
As of this week, about 1.45 million of the 2.5 million available doses of Pfizer have been ordered, and about 850,000 of available Moderna shots have been ordered, officials said. More orders are expected in the coming days.
Very young children are the last group of Americans who have not been recommended to get COVID-19 vaccinations. Up to about 20 million U.S. children under 5 would become eligible for vaccination if the government authorizes one or both shots.
It’s not clear how popular the shots will be. A recent survey suggests that only 1 in 5 parents of very young children would get their kids vaccinated right away.
And public health officials have been disappointed at how many older U.S. children, who have been eligible for shots for months, have yet to be vaccinated: Less than one-third of kids ages 5 to 11 have gotten the two recommended doses, according to government figures.
The news comes after months of anxious waiting by parents desperate to vaccinate their babies, toddlers and preschoolers.
“As we go down in the age groups, we see lower and lower uptake” of vaccines, said Dr. Lucia Abascal of the California Department of Public Health.
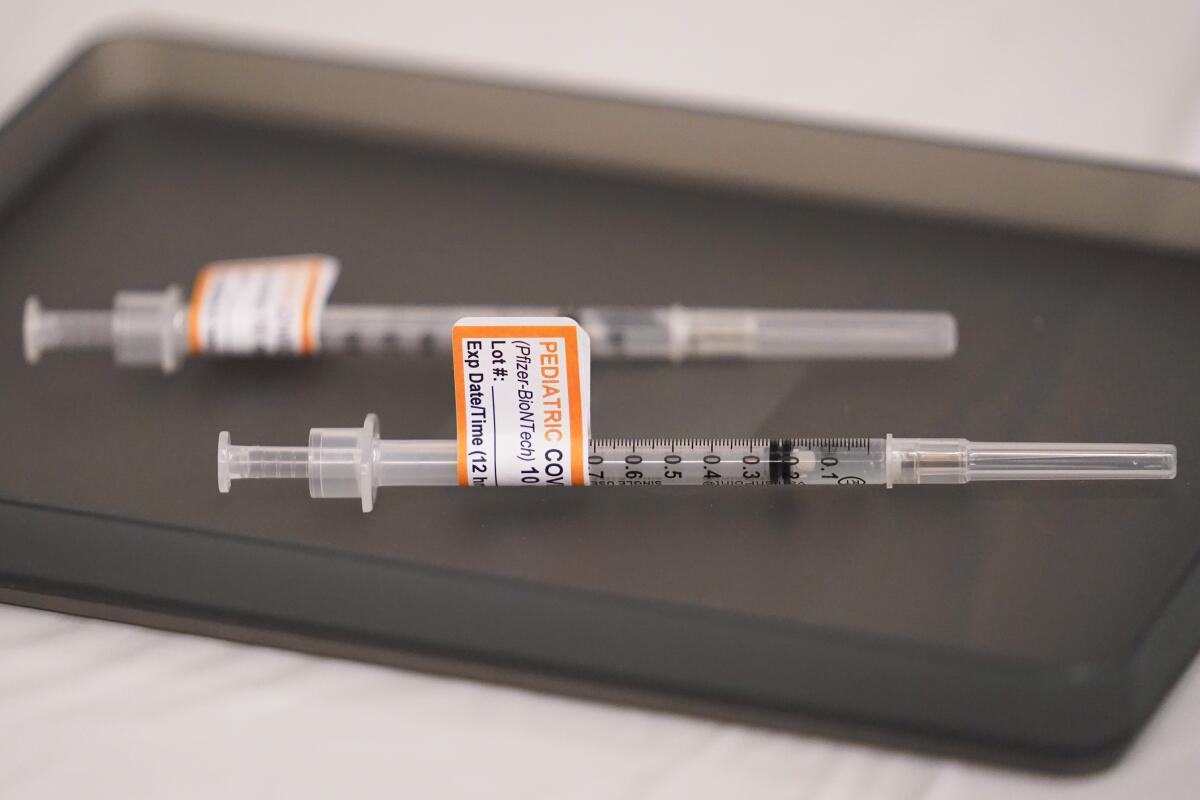
Pfizer has asked the Food and Drug Administration to authorize three doses of its COVID-19 vaccine for children aged 6 months to 4 years. Each dose is one-tenth of the amount adults receive.
Moderna has asked the FDA to authorize two shots for kids aged 6 months to 5 years, each containing a quarter of the dose given to adults.
The FDA authorizes the use of vaccines, while the Centers for Disease Control and Prevention issues recommendations to doctors and the public about using them.
Some public health officials are not convinced the state’s dip in coronavirus cases will stay consistent in the coming weeks.
An FDA advisory committee is scheduled to meet Tuesday and Wednesday to review data from the two companies. Officials say they expect a FDA decision shortly after that meeting.
A CDC advisory committee is scheduled for next Friday and Saturday, with a CDC decision expected soon after.
Vaccinations should begin in earnest as early as June 21, White House COVID-19 coordinator Dr. Ashish Jha told reporters last week.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.