Pfizer, U.S. sign $5-billion deal for 10 million treatment courses of COVID-19 pill

NEW YORK — The U.S. will pay Pfizer $5.29 billion for 10 million treatment courses of its experimental COVID-19 pill if regulators authorize the drug — the nation’s largest purchase agreement yet for a COVID-19 therapy.
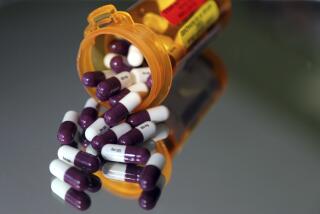
Pfizer asked the Food and Drug Administration on Tuesday to authorize emergency use of the pill, which has been shown to significantly cut the rate of hospitalizations and deaths among people with coronavirus infections.
The FDA is already reviewing a competing pill from Merck and will hold a public meeting on it later this month.
The price for Pfizer’s potential treatment amounts to about $529 per course. The U.S. has already agreed to pay about $700 per course of Merck’s drug, for about 3.1 million treatments in total.
Pfizer said Thursday that the price being paid by the U.S. government reflects the high number of treatment courses it has purchased through 2022.
President Biden said in a statement that his administration was taking steps to ensure that the treatments “will be easily accessible and free.”
Some places in Los Angeles city and county won’t let you in without proof of full COVID vaccination. Others will just ask you to wear a mask.
“This treatment could prove to be another critical tool in our arsenal that will accelerate our path out of the pandemic,” Biden said, adding that vaccines protecting against COVID-19 remain the strongest tool.
Pfizer is seeking approval for the treatment in several other countries, and there are advance purchase agreements with other governments as well.
On Tuesday, the company signed a deal a with United Nations-backed group to allow generic drugmakers to produce low-cost versions of the pill for certain low- and middle-income countries. Merck has a similar deal for its pill, which was authorized for use in Britain earlier this month.
Pfizer reported earlier this month that its pill cut hospitalizations and deaths by 89% among high-risk adults who had early symptoms of COVID-19.
A new study found a cheap antidepressant reduced the need for hospitalization among high-risk adults with COVID-19.
The company studied its pill in people who were unvaccinated and faced the worst risks from the disease because of age or health problems, such as obesity.
Pfizer wants the drug made available to adults who have mild-to-moderate COVID-19 and are at risk of becoming seriously ill. That’s similar to how other drugs are currently used to treat the disease. Currently, those FDA-authorized treatments must be administered intravenously or by injection given by a health professional at a hospital or clinic.
Pfizer’s pill is to be taken twice a day for five days, in combination with a second antiviral pill that boosts its effect.
Pfizer has already booked more than $24 billion in global revenue so far this year from Comirnaty, its COVID-19 vaccine, which has quickly become the drugmaker’s top-selling product.
Start your day right
Sign up for Essential California for the L.A. Times biggest news, features and recommendations in your inbox six days a week.
You may occasionally receive promotional content from the Los Angeles Times.
Pfizer and Merck are seeking approval for their treatments as COVID-19 cases start to rebound again in the U.S.
The seven-day rolling average for daily new coronavirus infections approached 87,000 on Wednesday, according to data from Johns Hopkins University. That’s up from around 68,000 late last month.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.