FDA narrows advice on prescribing controversial new Alzheimer’s drug
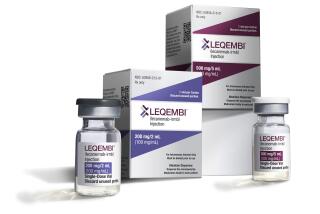
WASHINGTON — A month after approving a controversial new Alzheimer’s drug, federal health regulators Thursday signed off on new prescribing instructions that are likely to limit its use.
The Food and Drug Administration said the change is intended to address confusion among physicians and patients about who should get the drug, which has faced an intense public backlash since its approval last month.
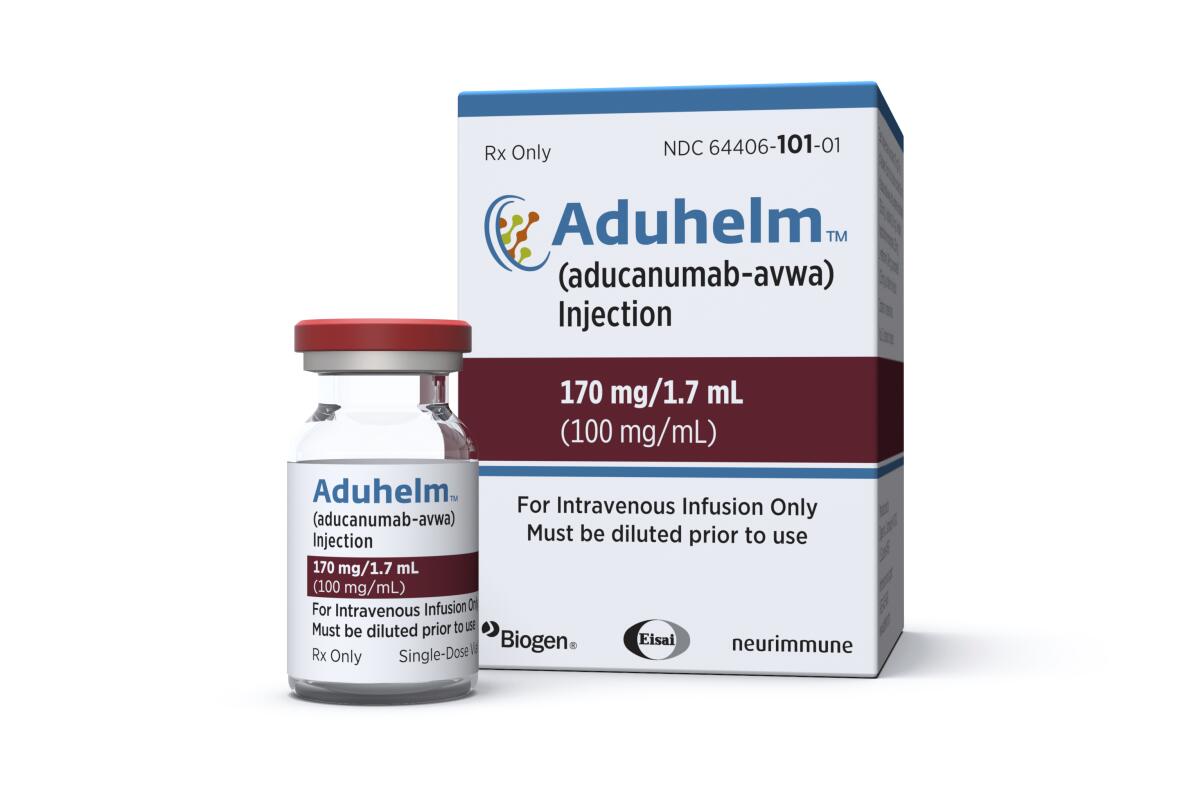
The new drug label emphasizes that the drug, Aduhelm, is appropriate for patients with mild symptoms or early-stage Alzheimer’s but has not been studied in patients with more advanced disease. That’s a big change from the original FDA instructions, which said simply that the drug was approved for Alzheimer’s disease in general.
Drugmaker Biogen announced the change in a news release Thursday, stating that the update was intended to “clarify” the patient group studied in the company trials that led to the drug’s approval. The FDA first approached the company about narrowing the label and approved the language.
“Hearing these concerns, FDA determined that clarifications could be made to the prescribing information to address this confusion,” the agency said in a statement. Despite the update, the FDA added that “some patients may benefit from ongoing treatment” if they develop more advanced Alzheimer’s.
When the drug was first approved, a top FDA official told reporters that the drug was “relevant to all stages of Alzheimer’s disease.”
Surprise over the approval of a controversial new Alzheimer’s drug has turned to shock that it might take nine years to find out if it really works.
The FDA’s OK last month quickly sparked controversy over Aduhelm’s $56,000-a-year price tag and questionable benefits. Three of the FDA’s outside advisors resigned over the decision, which Harvard researcher Dr. Aaron Kesselheim called the “worst drug-approval decision in recent U.S. history.”
On Thursday, Kesselheim tweeted that the change in prescribing advice was “a welcome step” but added that the FDA and Biogen should do much more to combat “misperceptions about this drug.”
Sweeping changes to drug labels are rare, particularly only a few weeks after approval.
“It’s a responsible move by both the FDA and Biogen to maximize the safety while giving the drug the best chance to work,” said Dr. Ronald Petersen of the Mayo Clinic, who has consulted for Biogen and other drugmakers. The drug’s side effects include brain swelling and bleeding.
The lack of progress in developing an effective Alzheimer’s drug that will treat the disease once it arises suggests that we’ve been looking at this problem in the wrong way.
Aduhelm hasn’t been shown to reverse or significantly slow the disease. But the FDA said that its ability to reduce clumps of plaque in the brain is likely to slow dementia. Many experts say there is little evidence to support that claim.
Biogen is required to conduct a follow-up study to definitively answer whether the drug slows mental decline. Other Alzheimer’s drugs only temporarily ease symptoms.
Because of its price and broad approval, some analysts worry that Aduhelm could add tens of billions in new expenses to the U.S. healthcare system, particularly the federal government’s Medicare program. Alzheimer’s affects about 6 million Americans, the vast majority old enough to qualify for Medicare.
Two congressional committees in the House have launched an investigation into the FDA’s review of the drug. And lawmakers in the Senate have called for hearings into the drug’s cost and impact on federal spending.
The narrower label may ease some of those concerns by shrinking the number of patients likely to get the drug, which requires monthly IVs. Many hospitals have already stated that they plan to limit the drug’s use to patients with earlier-stage disease. Doctors could still prescribe the drug for more advanced patients, though insurers might refuse to pay for it, citing the FDA label.
“It was pretty troubling that the previous label was so broad and included groups of patients in whom the drug had never been tested,” said Dr. Suzanne Schindler of Washington University in St. Louis. “I think this is a positive change because it better reflects the patients in whom the drug was actually studied.”
Wall Street analysts said the change wouldn’t significantly affect projected sales for Biogen. Michael Yee of Jefferies said in a research note that the company already planned to target the drug for the 1 million to 2 million Americans with mild Alzheimer’s.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.