EU says ‘no evidence’ to restrict use of AstraZeneca’s COVID-19 vaccine
BERLIN — The head of the European Union’s drugs regulator says there is “no evidence” that would support restricting the use of AstraZeneca’s COVID-19 vaccine in any population, as Germany and a handful of other European countries have done amid concerns over rare blood clots in people who got the shot.
Emer Cooke, executive director of the European Medicines Agency, said Wednesday that her Amsterdam-based agency continues to study reports of new cases as they come in and will provide a further assessment next week.
On Tuesday, an independent vaccine expert panel in Germany said AstraZeneca shots should not routinely be given to people younger than 60 because of a rise in reported cases of unusual blood clots in the days after vaccination. The German government followed the recommendation and said the vaccine would be prioritized for people 60 and older, although exceptions can be made in consultation with doctors.
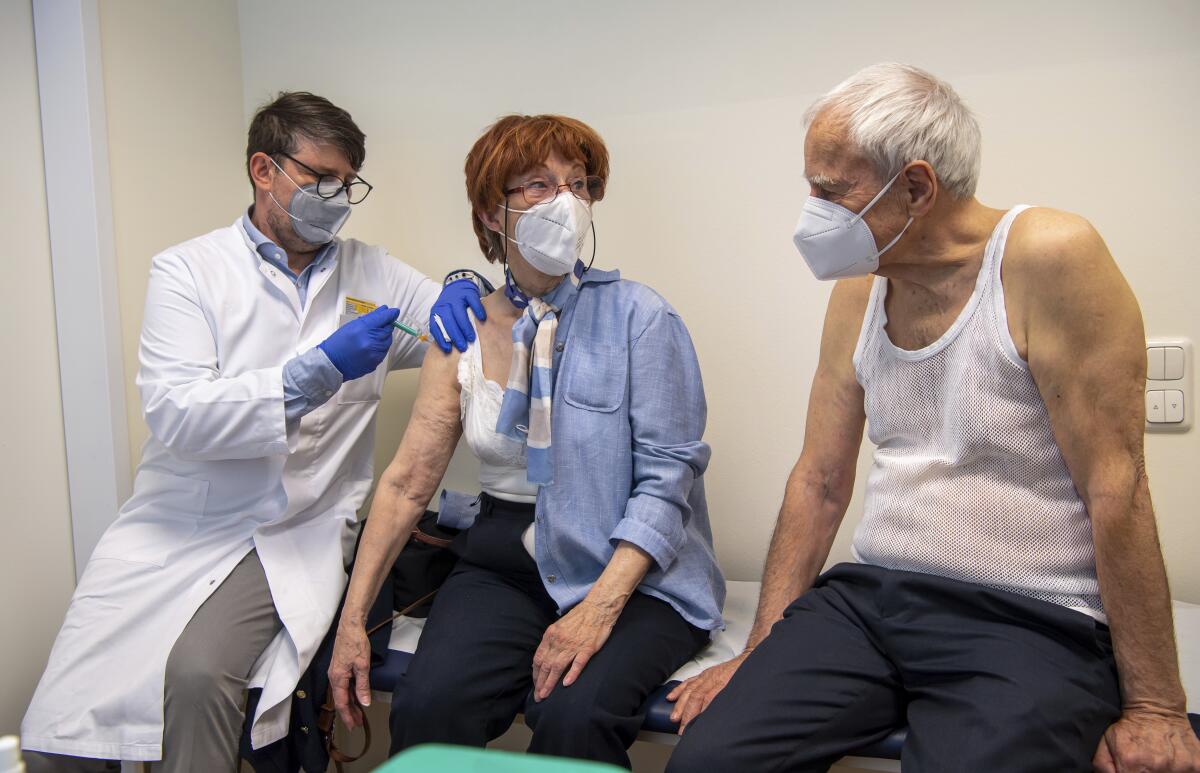
On Thursday, German President Frank-Walter Steinmeier was vaccinated with the AstraZeneca shot to try to boost confidence in the vaccine. The presidential office said in a statement that Steinmeier, 65, received his first shot at a hospital in Berlin on Thursday.
“According to the current scientific knowledge, there is no evidence that would support restricting the use of this vaccine in any population,” Cooke told reporters Wednesday.
Last month, the European Medicines Agency, which authorized the AstraZeneca vaccine in January, said the vaccine’s benefits outweigh any risks. The agency issued the guidance after some European countries, including Germany and France, suspended its use over blood clot fears.
German officials have decided to limit the use of AstraZeneca’s coronavirus vaccine in people younger than 60 after more unusual blood clots were reported.
Cooke said the agency’s assessment was based on 62 cases worldwide of unusual blood clots, including 14 deaths, reported to the EMA by March 22. Those figures included a “significant” number of the cases reported in Germany but not all, she said.
German regulators said they had received 31 reports of rare blood clots in the head in recipients of the AstraZeneca vaccine and nine deaths up to March 29, roughly doubling the number that had been reported in the country by March 22.
Cooke said the EMA would include the Germany cases in its review, “and they will form part of the ongoing evaluation that the committee is undertaking, as will any additional cases that are reported from other countries and regions.”
Based on the numbers reported to the agency so far, there have been 4.8 cases of the rare blood clots per million doses of the AstraZeneca vaccine administered, she said.
The latest twist created another layer of uncertainty for a product already facing dwindling public support in Europe
For the Pfizer-BioNTech vaccine, “based on the same criteria, it was 0.2 cases per million,” Cooke added. “And for the Moderna vaccine based on the same criteria, zero cases per million. But that probably reflects that there’s a lot less use of Moderna at the moment in Europe.”
She said the EMA’s experts so far had not determined any underlying risk factors for the blood clots or established a causal relationship with the AstraZeneca vaccine.
“The link is possible, and we cannot say any more than that at this point,” Cooke said.
Among those affected by Germany’s decision to halt the AstraZeneca vaccine’s use in people younger than 60 were tens of thousands of teachers, who had only recently been offered appointments for the shot.
The head of the German Teachers’ Assn., Heinz-Peter Meidinger, described the decision as a “catastrophic setback” and called for school staffers to be offered another type of vaccine instead.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.