Pfizer seeks to expand Omicron booster to 5- to 11-year-olds
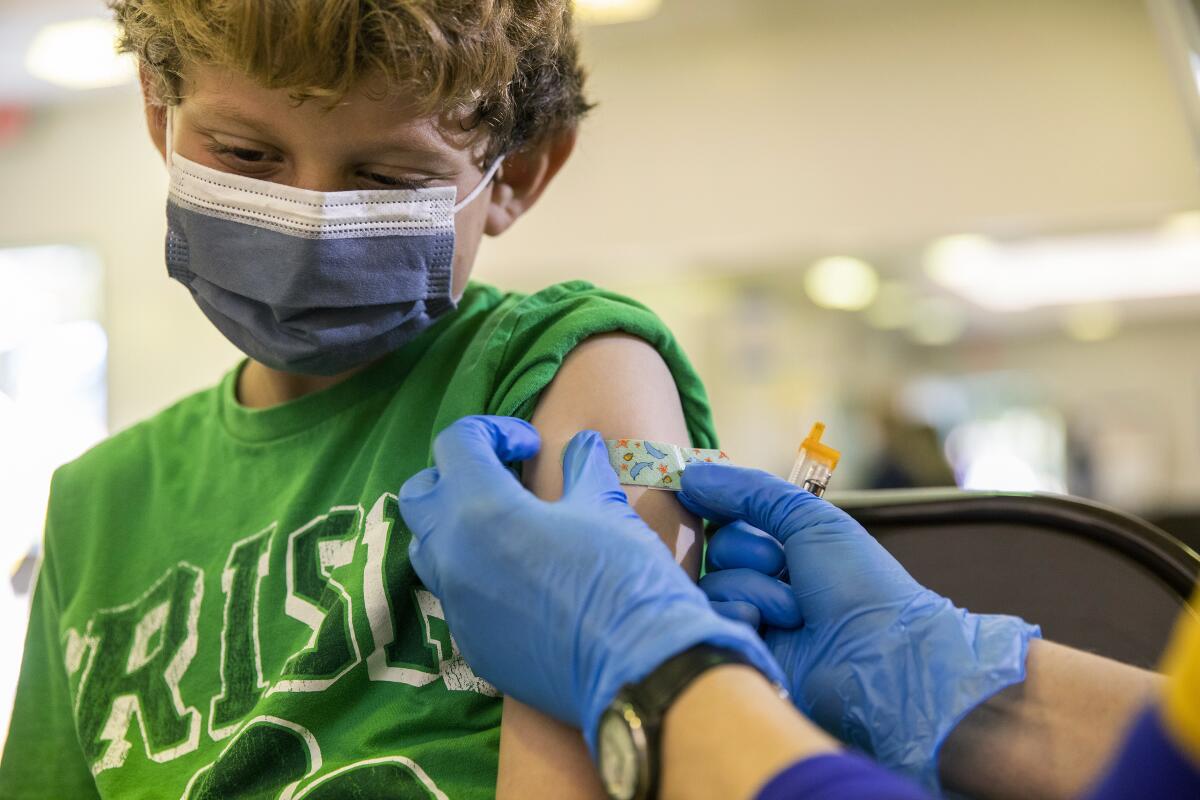
Pfizer asked U.S. regulators Monday to authorize use of its updated COVID-19 booster shot for children ages 5 to 11.
Elementary school-age children are already eligible for kid-size doses of Pfizer’s original vaccine, which are one-third the dose given to people 12 and older. If the Food and Drug Administration agrees, they would get a kid-size dose of the new Omicron-targeted formula when it is time for their booster.
FDA vaccine chief Dr. Peter Marks said last week that he expected a decision on boosters for 5- to 11-year-olds soon.
Pfizer and partner BioNTech also announced a new study of the Omicron-focused booster in even younger children, those ages 6 months through 4 years. The study is designed to test different doses.
A big question with the new COVID-19 vaccine: Can you mix brands? Here is what experts say
Updated boosters made by both Pfizer and Moderna rolled out this month for everyone 12 and older. They’re essentially a tweak to the vaccines that already have saved millions of lives — a combination or “bivalent” shot that protects against the original coronavirus strain as well as the BA.4 and BA.5 Omicron subvariants responsible for most of today’s COVID-19 cases.
The hope is that the modified boosters will help continue the downward trend in new cases and blunt another winter surge. The Centers for Disease Control and Prevention says 4.4 million Americans have gotten a bivalent booster so far.