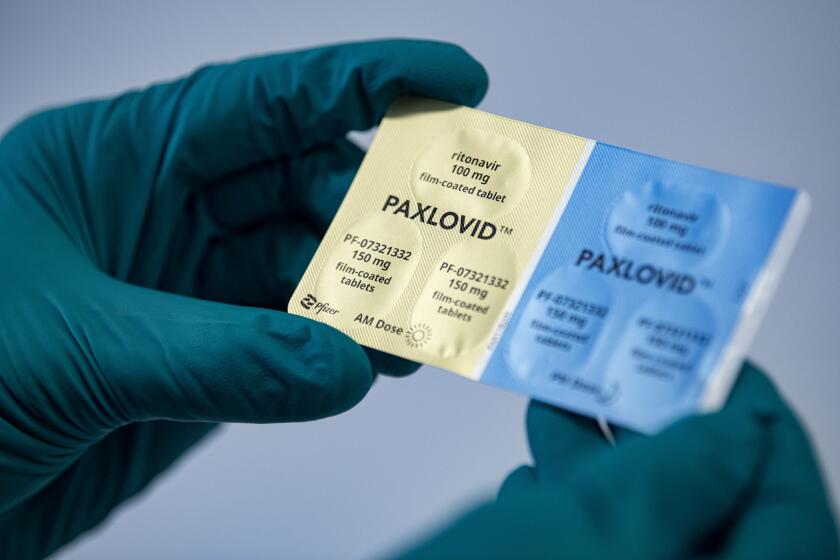
FDA allows pharmacists to prescribe Pfizer’s Paxlovid COVID pill

WASHINGTON — Pharmacists can prescribe the leading COVID-19 pill directly to patients under a new U.S. policy announced Wednesday that’s intended to expand use of Pfizer’s drug Paxlovid.
The Food and Drug Administration said pharmacists can begin screening patients to see if they are eligible for Paxlovid and then prescribe the medication, which has been shown to curb the worst effects of COVID-19. Previously, only physicians could prescribe the antiviral drug.
Biden administration officials have expressed frustration that several hundred Americans continue to die of COVID-19 daily, despite the availability of vaccines and treatments.
Administration officials have been working for months to increase access to Paxlovid, opening thousands of sites where patients who test positive can fill a prescription for the drug. The FDA change will make thousands more pharmacies eligible to quickly prescribe and dispense the pill, which must be used early to be effective.
“Since Paxlovid must be taken within five days after symptoms begin, authorizing state-licensed pharmacists to prescribe Paxlovid could expand access to timely treatment,” said FDA drug center director Patrizia Cavazzoni.
Still, use could be limited by paperwork requirements. Patients are expected to bring their recent health records — including blood tests — and a list of their current medications so pharmacists can check that Paxlovid won’t negatively interact with other drugs. As an alternative, pharmacists can consult with the patient’s doctor.
There are two antiviral pills available for eligible patients who have recently tested positive for the coronavirus. And they’re free.
Paxlovid is intended for people with COVID-19 who are more likely to become seriously ill. That includes older people and those with other health issues like heart disease, obesity, cancer or diabetes that make them more vulnerable.
The FDA authorized Paxlovid to treat COVID last December based on results showing it reduced hospitalizations and deaths by nearly 90% among unvaccinated patients most likely to get severe disease. The drug has shown less impressive results in patients who already have vaccine protection, and some physicians have reported cases of COVID-19 symptoms returning after treatment with the drug.
Expanding the so-called test-and-treat program to include pharmacists could add thousands of additional options for patients. The two biggest U.S. drugstore chains — CVS Health and Walgreens — run around 19,000 locations combined.
CVS Health already provides COVID-19 care at 1,100 clinic locations inside drugstores.
Health experts are urging people to be cautious if they develop symptoms again after taking anti-COVID pills.
There also are nearly 19,400 independent pharmacies not tied to a big chain, according to the National Community Pharmacists Assn.
Pharmacist Michele Belcher of Grants Pass, Ore., said she hoped to be able to test customers for COVID-19 and offer the pill because there is a shortage of primary care doctors in her community.
Belcher said she worries that some people may have a hard time getting a doctor’s appointment for a prescription during the narrow window of time available to start the medication.
Belcher, owner of the independent Grants Pass Pharmacy, said she used to test and treat for COVID-19 using injectable drugs that are no longer as effective.
One month into its “test-to-treat” initiative, state health workers are slow to get Pfizer’s Paxlovid and Merck’s molnupiravir to patients.
Her pharmacy routinely checks for potentially harmful interactions with other drugs a patient may be taking, she said.
“Pharmacists are the drug experts,” she said. “That’s something we do every day, all day, make sure there are no interactions with any medications.”
Murphy reported from Indianapolis.