Is COVID-19 vaccine going to waste due to FDA safety rules?

President Biden has promised enough COVID-19 vaccine to immunize every willing adult by the end of May. But right now, the gap between supply and demand is so large that vaccine administrators want to extract every last drop from each vaccine vial — if federal regulators will let them.
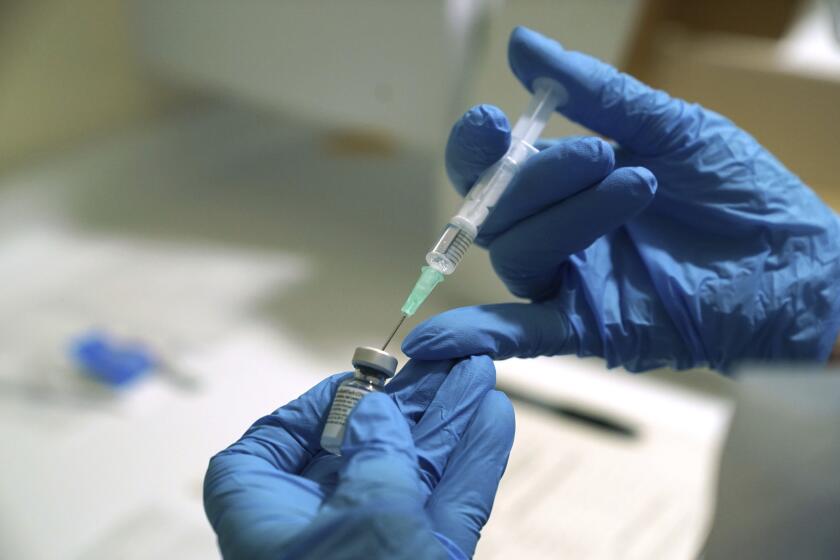
Pharmacists say it’s common to have half a dose left in a vial of Pfizer and BioNTech’s vaccine after five or even six doses have been administered — and to have half a dose left after 10 doses have been drawn out of a Moderna vial. Combining two half-doses of the same vaccine could increase the number of available shots by thousands, they argue.
They’d like permission to use a single hypodermic needle to withdraw leftover vaccine from two vials after all the full doses have been removed. The American Society of Health-System Pharmacists asked the U.S. Food and Drug Administration consider this request in a recent letter.
The governors of Colorado and Oregon also have sought permission to allow their pharmacists to pool leftover vaccine from not-quite-empty vials.
Federal health regulators, however, have long opposed this for safety reasons.
Each time a fresh needle is inserted into a vial, there’s a theoretical chance for contamination. But because we’re in the midst of a public health emergency, the FDA allows COVID-19 vaccine administrators to insert up to six different needles into a single vial to draw out six doses. Each draw presents a slightly higher risk than the one before it.
A dose that results from a seventh draw from two separate vials would have 14 times the risk of the first dose from a single vial. That’s more risk than the FDA has been willing to accept.
The FDA wouldn’t comment on the pharmacists’ request but reiterated that its current policy is that “doses not be pooled from different vaccine vials, especially for coronavirus vaccines, which are not formulated with a preservative.”
Mixing and matching doses of different COVID-19 vaccines was an idea that scientists dismissed out of hand. Now they’re taking it seriously.
The website for the Centers for Disease Control and Prevention explicitly tells vaccinators to discard vials “when there is not enough vaccine to obtain a complete dose. Do NOT combine residual vaccine from multiple vials to obtain a dose.”
Ann Marie Pettis, president of the Assn. for Professionals in Infection Control and Epidemiology, said this advice was sound. There is always a tiny chance that one of the two vials has previously been contaminated, and any shot that combined their contents would be contaminated as well, she said.
“It’s a recipe for disaster,” Pettis said.
Spokespeople for both Pfizer and Moderna said excess portions of their vaccines must be discarded and never pooled. Johnson & Johnson had no comment on the issue.
Before the COVID-19 vaccines arrived, public health officials generally frowned on giving multiple patients doses of medicine from the same vial unless it contained an antibacterial preservative. Most children’s vaccines, for example, have been shipped and stored in syringes or single-dose vials since 2001, when drug companies stopped using a preservative containing traces of mercury.
A pair of studies in Science examine how coronavirus variants evolve in human hosts and why experts are concerned about relaxing restrictions too soon.
Rajesh Gupta, a biologics consultant who set up a sterility testing lab while serving at the FDA’s Center for Biologics Evaluation and Research from 2006 to 2013, sees little risk in the way COVID-19 vaccines are administered, or in using a single needle to combine vaccine from two vials. The COVID-19 vaccines are going into arms so quickly after vials are removed from cold storage that there’s no danger of contamination, he said.
As long as the rubber stopper on the top of the vial is carefully wiped with disinfectant every time it’s about to be penetrated with a syringe, “I can say with some degree of confidence that it’s scientifically sound,” Gupta said.
Patricia Slattum, a retired Virginia Commonwealth University pharmacy school professor, has been volunteering at a mass vaccination site in Richmond, Va. She said she hopes the FDA will consider allowing vaccinators to draw the leftover vaccine from two vials.
“We who are doing this work all feel this pressure, that our doing it well is one of the ways we’re going to get out of this pandemic,” said Slattum. “You just don’t want to waste any vaccine!”
This story was produced by Kaiser Health News (KHN), a national newsroom that provides in-depth coverage of health issues and that is one of the three major operating programs at Kaiser Family Foundation KHN is the publisher of California Healthline, an editorially independent service of the California Health Care Foundation.