U.S. regulators post positive review of Pfizer vaccine data
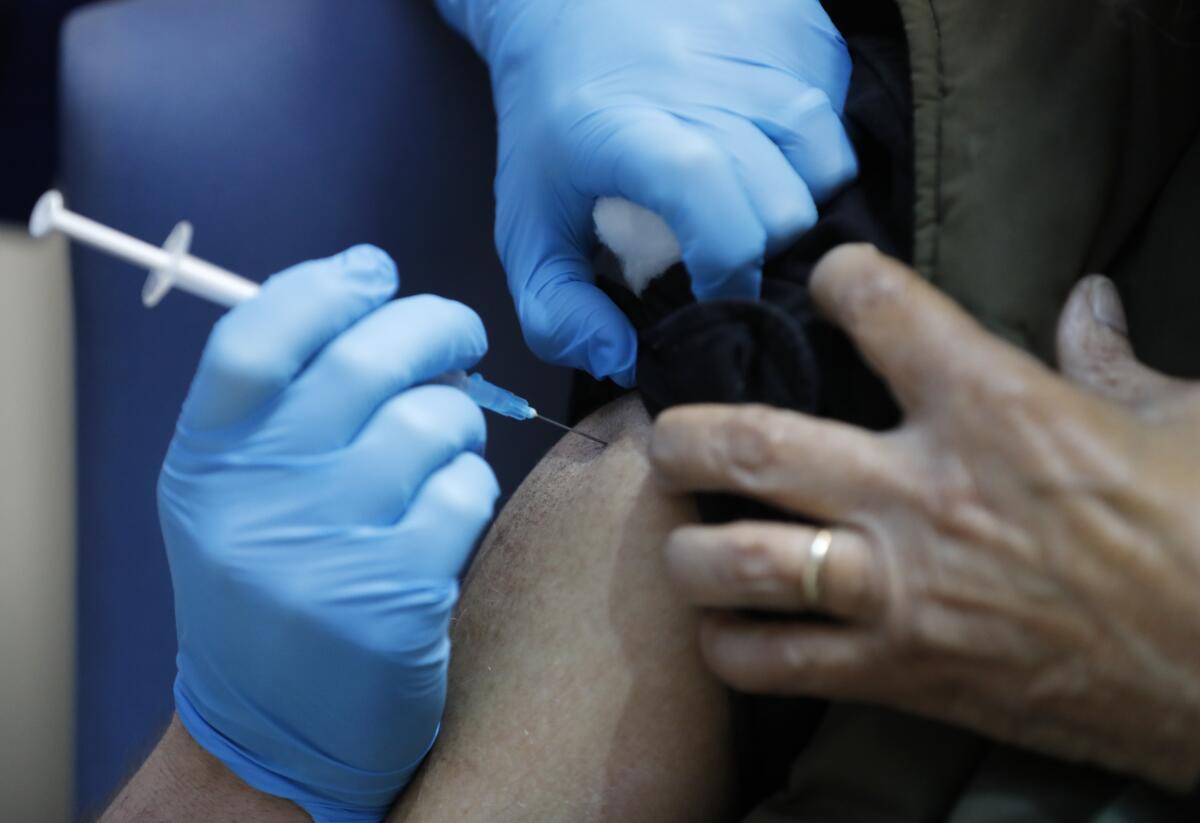
WASHINGTON — The coronavirus vaccine made by Pfizer Inc. and its German partner BioNTech SE is highly effective in preventing COVID-19 and there are no safety concerns that would prevent it from being granted an emergency-use authorization, according to a report by staff of the U.S. Food and Drug Administration.
The report offers a first look at the U.S. review of the vaccine ahead of a public meeting Thursday of outside advisors to the agency. The FDA could clear the shot shortly after the meeting, with as many as 6.4 million doses immediately available to kick off a vast immunization effort designed to end a pandemic that has killed more than 283,000 Americans.
The findings, posted on the agency’s website Tuesday, echoed previously disclosed data from the companies that showed the vaccine was 95% effective in preventing symptomatic COVID-19. It also said that two months of trial data revealed no significant safety issues.
Pfizer requested an emergency authorization in the U.S. on Nov. 20. Regulators in the U.K. approved the vaccine earlier this month, and on Tuesday, the National Health Service there launched an ambitious vaccination campaign that will begin with tens of thousands of people over the age of 80 in the coming days. The shot is also under review in the European Union.
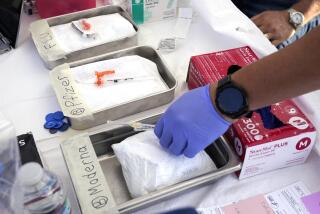
The vaccine uses technology called messenger RNA that transforms the body’s cells to fight the virus. Moderna Inc. also makes an experimental COVID-19 vaccine based on messenger RNA, and FDA advisors will meet next week to consider whether it should receive emergency authorization.
In their report, FDA staff wrote that analyses “showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19.” Common side effects included injection-site reactions, fatigue and headache.
Pfizer has said the shot protected people of all ages and ethnicities, but there isn’t enough data on children younger than 16 to determine whether it will work in pediatric populations, FDA staff said. Data is also insufficient to make any determinations about people who are pregnant, lactating or immunocompromised.
Among participants in the Pfizer clinical trial who didn’t show signs of COVID-19 infection during vaccination, there were eight virus cases among those who received the shot and 162 among those who got a placebo, according to the report.
It’s unclear whether the vaccine is effective at preventing COVID-19’s spread, according to the report. It’s possible the vaccine didn’t work as well at preventing cases where people didn’t have virus symptoms as it did at preventing symptomatic cases, meaning there will likely be a continued need for basic public-health measures.
“Asymptomatic cases in combination with reduced mask-wearing and social distancing could result in significant continued transmission,” FDA staff wrote.
If Pfizer’s vaccine is cleared by the FDA, Trump administration officials have said it could begin to be distributed within 24 hours. Between Pfizer and Moderna’s vaccines, 40 million doses are expected to be available by the end of the year, enough for 20 million people to get the two-shot regimen.
The Trump administration is confident that the U.S. will have enough supply to eventually vaccinate everyone, a senior administration official said Monday on a call with reporters, though only the shots made by Pfizer and Moderna are expected to be cleared by the FDA this month. The U.S. also has agreements to get shots from AstraZeneca Plc, Johnson & Johnson and others.
The Centers for Disease Control and Prevention’s immunization committee recommended healthcare workers and long-term care residents be first in line, totaling roughly 23 million people. It will be up to the states to determine exactly how to distribute the vaccines.