How L.A. doctors plan to include more people of color in COVID-19 vaccine trial
The patients at Dr. Eric Daar’s hospital are at high risk for becoming seriously ill from COVID-19, and he’s determined to make sure they’re part of the effort to fight the disease.
He also hopes they can protect themselves in the process.
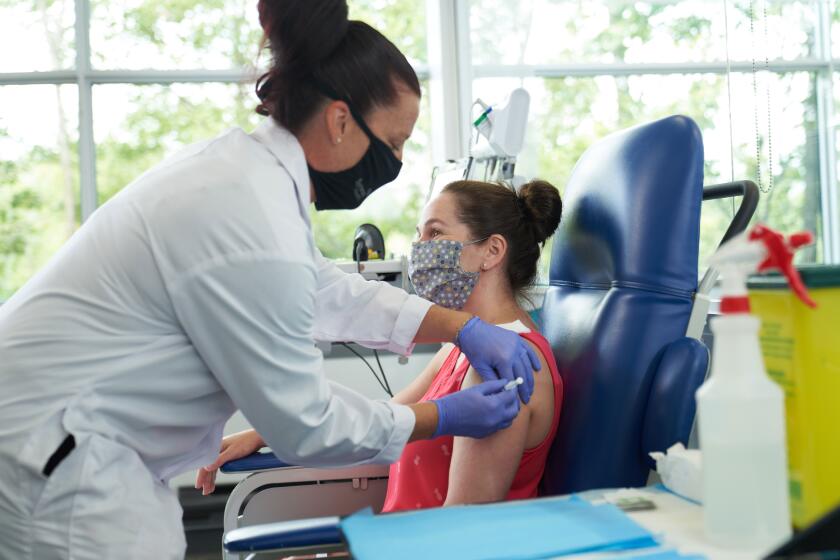
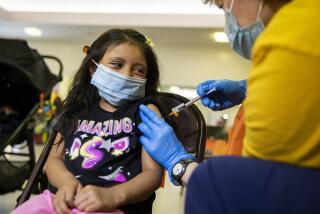
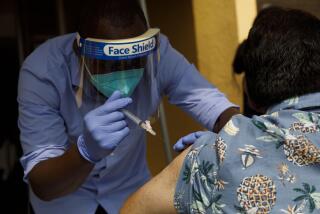
As Daar and his colleagues at Harbor-UCLA Medical Center begin enrolling 500 volunteers in a trial to test a COVID-19 vaccine produced by AstraZeneca, they will try to ensure that most, if not all, of them are people over 65, those with chronic illnesses and members of underserved racial and ethnic groups.
They know it won’t be easy.
“It’s a priority and obligation to make sure our community is well-represented in these trials,” said Daar, an infectious diseases specialist who dropped his other research projects to focus on a COVID-19 vaccine.
The safety-net hospital in Torrance serves patients in the South Bay who are predominantly Black, Latino and Pacific Islander. Many live in crowded homes and make their livings doing “essential” work that exposes them to the virus: they’re orderlies, cooks, house cleaners, day laborers, bus drivers and sanitation workers.
“If you don’t have a community represented in the trial, it’s hard to extrapolate your results to the community,” said Daar’s colleague, Dr. Katya Corado. “We want to find something to protect our patients and loved ones.”
Latino and Black residents in the United States are nearly three times more likely to be diagnosed with COVID-19 and nearly five times more likely to be hospitalized with the disease. In Los Angeles County, Latinos in particular have been disproportionately stricken by the virus.
Eight of 10 COVID-19 deaths nationwide are in people over 65, according to the Centers for Disease Control and Prevention.
Historically, Black and Latino patients have been less likely to be included in clinical trials for disease treatment, despite federal guidelines that encourage minority and elder participation.
The National Institutes of Health and the Food and Drug Administration have urged infectious disease researchers to focus on these vulnerable populations in the large phase 3 trials that will test how well vaccines prevent COVID-19.
The NIH has launched studies of the disproportionate impact of COVID-19 on Black and Latino Americans, hoping to prepare the country for the next epidemic.
Harbor-UCLA, a public teaching hospital that is owned and operated by Los Angeles County, is one of roughly 100 sites across the country testing the AstraZeneca vaccine candidate, which was developed in collaboration with Oxford University in Britain. Phase 3 trials for vaccine candidates produced by Moderna and Pfizer are already underway. Each of the three companies is seeking to recruit 30,000 people — 20,000 of whom will get the vaccine and 10,000 a placebo — to test whether the vaccine prevents COVID-19.
According to the AstraZeneca trial protocol, patients will have to make 15 to 20 visits to the hospital during the course of the two-year trial. For each visit, they’ll receive up to $100.
The Harbor-UCLA team is reaching out to prospective participants by distributing leaflets to clinics and community organizations, and by creating targeted social media campaigns, Daar said. The hospital will offer car services to bring them to their appointments.
USC’s Keck School of Medicine is also participating in the AstraZeneca trial, and has placed a recruitment site in Vernon to help reach more vulnerable populations. The city south of downtown Los Angeles is home to many factories and meatpacking plants where workers have experienced high rates of coronavirus infection.
Recruitment of high-risk patients in other COVID-19 trials so far has been mixed. Moderna, which began the first phase 3 trial on July 27, announced Friday that 18% of its 13,000-plus enrollees so far were of Black, Latino or Native American heritage — a high percentage as clinical trials go, but only about one-third of the goal set by NIH officials.
Clinicians suspect that the higher rates of disease and hospitalization in minority groups are due both to health conditions — such as under-treated diabetes and heart disease — and to higher exposure in workplaces and crowded housing. Environmental factors like polluted neighborhoods could also have an impact.
While there’s little evidence that vaccines affect Black or Latino people differently than white people, the subject hasn’t really been studied, said Dr. Akilah Jefferson Shah, an allergist/immunologist and bioethicist at the University of Arkansas for Medical Sciences. That’s another reason for making sure these groups are well-represented in trials, she said.
“We know now there are subgroup responses to drugs by sex, but no one figured it out until they started including women in these studies,” Jefferson Shah said. “Race is not genetic. It’s a social construct. But there are genetic variants more prevalent in certain populations. We won’t know until we look.”
The first wave of coronavirus vaccines might be like a flu shot, experts say, curbing symptoms in some patients but not protecting them from COVID-19.
Enrolling a diverse group of patients will also help build trust in and uptake of a COVID-19 vaccine, Corado said. In a poll conducted in May by The Associated Press-NORC Center for Public Affairs Research, just 25% of Blacks and 37% of Hispanics said they would definitely seek out the vaccine, compared with 56% of whites.
In July, the UCLA vaccine team began holding weekly Zoom meetings with about 25 activists and clergy members to learn what people in their communities were saying about the vaccine, and to get tips on how to design educational materials for the trial.
What they’ve heard suggests they’ll have an uphill recruitment battle.
One member of the community council, HIV activist Dontá Morrison, noted that people frequently say on social media that the vaccine is designed to spread COVID-19 as part of a plot to get rid of black voters. (None of the vaccines contains infectious coronavirus.)
“It may seem far-fetched, but those are the conversations because we have an administration that has not shown itself to be trustworthy,” Morrison said.
He noted that the first challenge Harbor-UCLA recruiters face is convincing community leaders, particularly clergy members, that the vaccine is safe. Church leaders worry they’ll be blamed for supporting the trial if the vaccine ends up making their congregants sick, he said.
If done right, the trial could build trust in medical science while helping minorities help themselves — and the rest of us — find a way out of the current mess, Morrison said.
Dr. Raphael Landovitz, a doctor and research scientist at the Ronald Reagan UCLA Medical Center in Westwood, which is also participating in the trial, agreed.
“We’re hoping that people understand this is a chance — if we succeed — to take back some power and control in this situation that has made so many of us feel so powerless,” he said.
Arthur Allen writes for Kaiser Health News, a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation and is not affiliated with Kaiser Permanente.