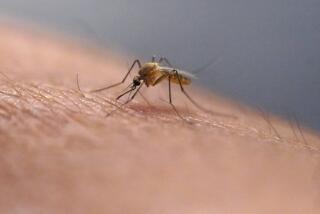
Scientists aim to fight malaria with genetically engineered mosquitoes
Watch out, malaria — scientists are coming for you with genetically engineered mosquitoes.
A team of researchers has altered the DNA in mosquitoes so they can fight and kill the parasites that cause malaria inside their own bodies. That way, the insects can’t transmit the deadly parasites to humans.
And for the first time, scientists have armed these malaria-killing mosquitoes with another genetic superpower — the ability to pass on their disease-destroying genes to 99.5% of their offspring.
“In principal, if you have a population of 100 million mosquitoes and you released 1 million of these genetically modified mosquitoes into the same area, the new gene would be spread throughout the entire population in less than a season,” said Ethan Bier, a biologist at UC San Diego who helped create the malaria-proof mosquitoes.
A report on the insects was published this week in the journal Proceedings of the National Academy of Sciences.
Malaria is caused by a handful of Plasmodium parasites that infect human blood cells and then travel to other parts of the body, causing symptoms such as fever, vomiting, seizures, coma and even death.
Mosquitoes get the parasites by feeding on blood from infected humans, then spread it to other humans during subsequent meals.
However, if the mosquitoes could kill the parasites, they wouldn’t spread from person to person. That would bring the world one step closer to eradicating a disease that kills nearly 600,000 people each year.
The researchers set out to do this by tinkering with the DNA of Anopheles mosquitoes, the ones that spread malaria parasites.
It began with UC Irvine biologist Anthony James, who has spent 20 years engineering mosquitoes that are resistant to diseases like malaria and Dengue fever.
He and his colleagues identified a set of genes in mice that allow them to mount a strong immune response to the parasites that cause malaria in humans. When the scientists inserted these mouse genes into mosquito DNA, the resulting insects were able to resist infection by the Plasmodium falciparum parasite. James’ team reported that feat in a 2012 study in the Proceedings of the National Academy of Sciences.
However, the mosquitoes could pass on their new malaria-resistance genes to their offspring only about half of the time.
“It had to be better than that,” James said.
Enter Bier from UC San Diego and his graduate student Valentino Gantz. Last summer, Gantz built a set of genes that used the powerful gene-editing tool known as CRISPR/Cas9. With this “gene drive” system, engineered fruit flies were able to pass on a spliced-in trait 97% of the time, instead of the expected 50%. Gantz and Bier published a report on their work in March in the journal Science.
When they saw how successful it was, they started looking for other applications for their new tool.
James’ mosquitoes were an appealing choice. Among other things, they liked the fact that James’ malaria-fighting strategy relied on making the insects resistant to the parasite instead of simply killing them off.
“Some people had proposed knocking down genes for survival and reproduction, which could lead to the eradication of the species,” Bier said. “We felt more comfortable with the softer approach. James doesn’t harm the mosquitoes.”
That’s not just nice for the mosquitoes, it’s better for the environment, according to Gregory Lanzaro of UC Davis, who studies how diseases spread via insects. If the mosquitoes were wiped out, they would leave a hole in the ecosystem that might be filled by a pest that’s even more dangerous, among other potential ripple effects.
It took Gantz three months to create a gene drive for the mosquitoes that included the anti-malarial mouse genes that James sent down to San Diego. When it was done, Gantz sent it up to James’ lab in Irvine. The team there inserted it into 680 mosquito larvae.
Among those larvae, 251 survived to adulthood and mated with wild mosquitoes. Those pairings generated 25,712 offspring — two of which had the engineered gene.
But those two were enough. Between them, they fathered 143 mosquitoes that grew to adulthood, and about half of them had the desired genes. The carriers then bred with more wild mosquitoes, and they produced nearly 6,000 offspring. This time, more than 99% of them had the malaria-resistance genes.
“To get that primary event was difficult, but the secondary part was fantastic,” James said.
Lanzaro, who was not involved in the study, called the results “very encouraging.” Still, he noted that “a significant amount of work still needs to be done before this system produces a modified mosquito ready for field trials.”
The researchers agreed that they still have a long way to go before they release any of their engineered insects into the wild. Among other things, they would have to be certain that the malaria-resistance genes spread reliably from each generation to the next. They’d also want to make sure that the people who would live among the mosquitoes were OK with them.
“We could do all this stuff in the laboratory, but there has to be a regulatory structure in place before we send them off somewhere,” James said.
Science rules! Follow me @DeborahNetburn and “like” Los Angeles Times Science & Health on Facebook.
ALSO
Why the FDA doesn’t want you to say ‘GMO’
Dangerous dirt: Why the animal world is obsessed with staying clean
For face transplant recipients, some of the healing is psychological