A ‘rescue pen’ for opioid overdose gets FDA’s blessing
Opioid drug overdoses, the cause of some 16,000 fatalities and half a million emergency-department visits yearly, may have met their match: The Food and Drug Administration on Thursday approved the sale, by prescription, of a hand-held auto-injector of the opioid-reversal drug naloxone, a “rescue pen” that caregivers or family members can use to avert a potentially fatal overdose.
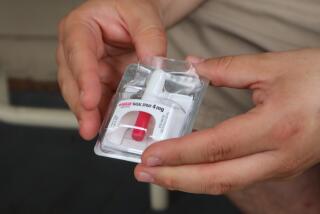
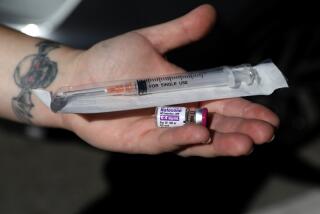
Delivered by syringe, naloxone has been a workhorse drug in emergency departments battling the relentless rise of opioid abuse in the last decade. The FDA’s blessing, granted after a deliberation of just 15 weeks, marks the first time the medication is to become available in a prefilled hand-held auto-injector to be used outside a hospital setting.
The device, to be marketed under the commercial name Evzio and produced for the Richmond, Va., firm Kaleo Inc., is modeled on devices like those that deliver epinephrine to patients with anaphylactic reactions, or insulin to diabetics. But like an automated external defibrillator, the Evzio kit also provides verbal instruction in the use of the injection for caregivers or family members who recognize the dangerously slowed heartbeat, depressed respiration and loss of consciousness that suggests someone taking opioids has overdosed. A training device allows caregivers and family members to practice emergency administration of 0.4 mg. of naloxone, which can be injected into muscle or just under the skin.
Making this product available for use beyond an ambulance or hospital “could save lives by facilitating earlier use of the drug in emergency situations,” said Dr. Bob Rappaport, director of the division of anesthesia, analgesia and addiction products at the FDA’s Center for Drug Evaluation and Research.
The use of Evzio is not without risks. Among the opioid-dependent, a shot of naloxone can cause sudden and severe opioid withdrawal, which can cause nausea, vomiting, accelerated heart rate, increased blood pressure, seizures and cardiac arrest.
Repeat doses may be needed, as opioids can outlast naloxone’s reversal effects, and the FDA cautioned that Evzio is not a substitute for emergency medical care.
The FDA’s approval of Evzio was billed by the agency Thursday as part of a two-pronged approach to addressing opioid-drug abuse, an epidemic that has overtaken automobile crashes as a cause of death in the United States.
In recent years, the agency has sought to reduce the abuse potential of opioid analgesics and tightened rules on the prescribing of opioid pain relievers. However, the FDA recently approved marketing of a new extended-release opioid painkiller, Zohydro, in a formulation that activists, emergency physicians and the agency’s own advisory panel have warned could be diverted and misused.
Such decisions, FDA Commissioner Margaret Hamburg said Thursday, aim to “ensure continued access to effective and appropriate medications for millions of Americans currently suffering from pain.”
In a statement released alongside the agency’s approval of the naloxone rescue-injector, Hamburg called the goals of reducing opioid abuse while preserving patients’ access to pain relief “compatible,” and said that “actions to address one should not be at the expense of the other.”
“While the larger goal is to reduce the need for products like these by preventing opioid addiction and abuse, they are extremely important innovations that will help to save lives,” Hamburg said in a statement.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.