Kaiser launches COVID-19 vaccine trial for children 5 to 11 in Northern California
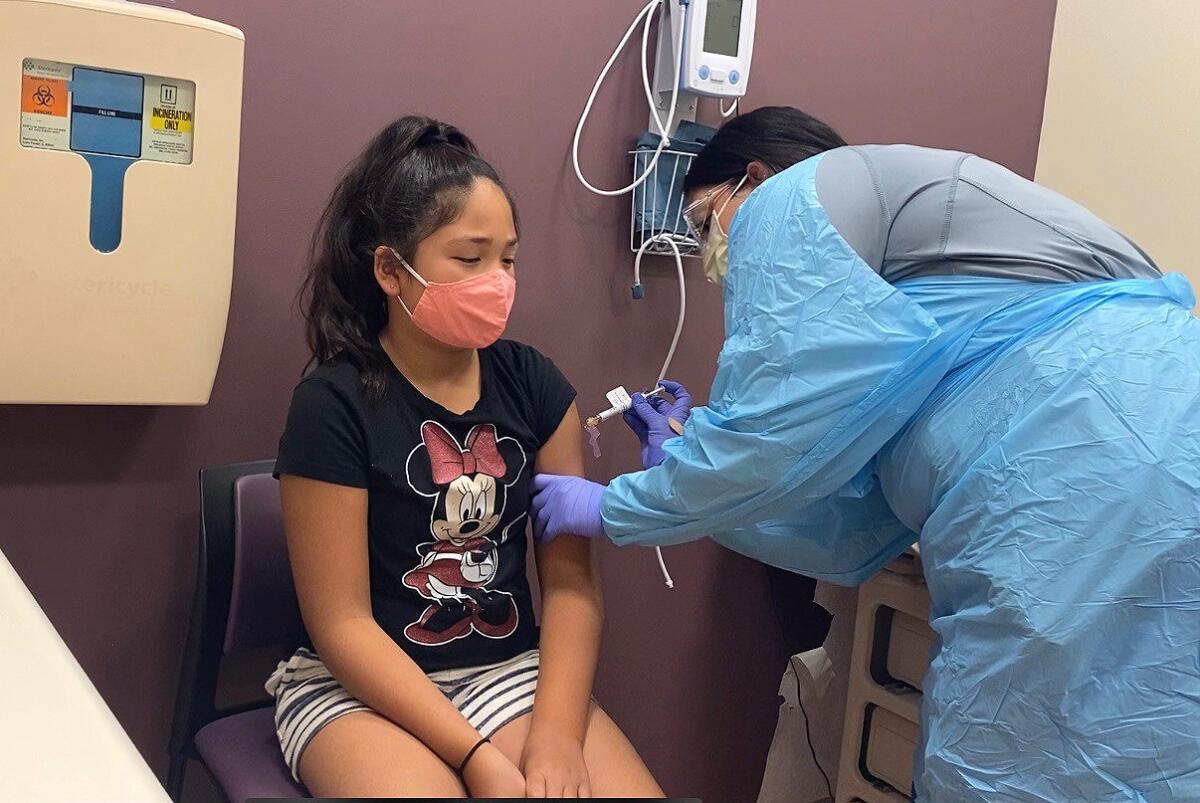
It’s been a hard year for 11-year-old Luci Guardino. Distance learning wasn’t easy, and she missed being able to have her friends over.
That’s why she’s participating in a COVID-19 vaccine trial for children ages 5 to 11 in Sacramento, which launched at a trio of Kaiser Permanente sites in Northern California this week. She wants to make sure she and others her age can go back to doing the things they enjoyed pre-pandemic.
“I definitely want to join the study to help make sure that we get back to normal life,” Luci, the daughter of a Kaiser pediatrician, said at a news conference Tuesday.
Luci is one of 75 children expected to be enrolled in the study, which is also being conducted at Kaiser sites in Oakland and Santa Clara. It’s part of a nationwide effort involving about 4,600 children to evaluate a vaccine being developed by Pfizer-BioNTech, sponsors of the trial.
A few weeks ago, Kaiser’s Los Angeles Medical Center kicked off a trial evaluating a Moderna vaccine for children 6 months to 11 years old, a Kaiser spokesperson said. So far, it has enrolled participants as young as 6.
At the Northern California sites, two-thirds of the participants will receive the vaccine, while the other one-third will receive a placebo. Designed as what’s known as a double-blind study, neither the clinicians nor the patients will know whether the vaccine is in the syringe. Clinicians will collect data on the effectiveness and safety of the dosage, including side effects.
After getting her first shot, Luci said her arm felt heavy, but she did not feel sick or have other adverse symptoms.
If all goes well with the trial, the vaccine could become available for young children as early as fall, according to Dr. Nicola Klein, director of the Kaiser Permanente Vaccine Study Center, which is conducting the trial.
“So it may be around the start of school,” Klein said, adding that the timing is a best-case scenario and ultimately is still “up in the air.”
Pfizer has a COVID-19 vaccine approved for children 12 and over. In April, Stanford began testing the Pfizer vaccine on children as young as 2. Moderna announced its vaccine trial for children 6 months to 11 years old in March.
U.S. expands use of Pfizer’s COVID-19 vaccine, paving the way for children as young as 12 to get shots.
COVID-19 vaccines in general have proved exceedingly safe among the more than 300 million Americans who have received one, but it’s still important to test them separately in younger age groups, according to Dr. Pia Pannaraj, an infectious disease specialist with Children’s Hospital Los Angeles.
“Children are not little adults, so we can’t assume that a vaccine that works well in adults will work in children,” said Pannaraj, who is also an associate professor of pediatrics at the hospital.
If the vaccine is approved for younger age groups, Pannaraj said the vast majority of children should get it. An allergy to vaccine components is one of the few contraindicators to receiving a shot, she said.
Children in general do not become as sick from coronavirus infections as adults, and older adults in particular. However, some do become ill enough to require hospitalization and intensive care. A small minority develop a serious complication not seen in adults called multisystem inflammatory syndrome, or MIS-C, essentially an inflammation of their entire body.
Besides possibly preventing serious complications, vaccinated children won’t be able to spread the virus to others who might fare worse, such as their parents, grandparents or teachers, Pannaraj said.
It also will help get kids like Luci back to normal.
“Children need to be able to go back to school and participate in sports, and it’s summer — have pool parties and slumber parties,” she said, “so it’s really important that they get vaccinated and be protected to be able to do all those things.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.