From botox clinics to wellness spas, antibody tests become the rage. Are they the real thing?

At a flotation therapy studio in Marina Del Rey, the sensory deprivation tank is empty, but antibody tests for the coronavirus are selling at $149. Customers of a botox clinic in Venice don’t even have to get out of their cars to get tested; a worker collects blood samples with the prick of a finger.
A banner for a clinic in Las Vegas advertises antibody tests and throws in a doctor visit for $169. And $125 antibody tests have recently been added to the menu at a chiropractic clinic in Florida that also offers libido-boosting vitamins and nonsurgical face-lifts.
The test-takers, from nurses and firefighters to the unemployed, seek a sense of certainty. They ache to know whether a flu they had was in fact COVID-19, the disease caused by the novel coronavirus. Some hope a positive result means they can throw out their masks. Others want certificates showing employers they are immune and can go back to work.
Not so fast, experts say. In the time of pandemic, opportunities can swing open for the nimble entrepreneur: storefront clinics and fancy spas once dedicated to beauty and wellness treatments now offer peace of mind through antibody testing. But misplaced trust in the tests, expert say, could be jeopardizing public health at large.
The tests don’t necessarily tell people whether they are immune to COVID-19 because scientists haven’t confirmed whether the antibodies even prevent re-infection. Most of the more than 200 tests on the market haven’t been cleared by regulators, and many could be faulty or of unknown origin, experts say. Across the U.S., several sites have been shut down for offering dubious antibody tests.
For several weeks, the Food and Drug Administration had allowed manufacturers to market antibody tests without supplying evidence that they are accurate. There is no FDA-approved test for antibodies related to COVID-19. And only 12 tests have been granted emergency authorization, which meets a lower standard.
Last week the agency, after heightened scrutiny from lawmakers, tightened its policies on unregulated tests, requiring manufacturers to promptly send in data proving their products are effective and apply for emergency authorization. At the flotation therapy studio, botox clinic and chiropractic clinic, the tests being offered are under review for such authorization.
Jim Flanigan, executive vice president of the American Society for Clinical Laboratory Science, expressed concern about the confusing nature of the regulations. “It truly could be snake oil,” he said of antibody tests that don’t have FDA authorization. “Without that, people can claim whatever they like and there seems to be no recourse.”
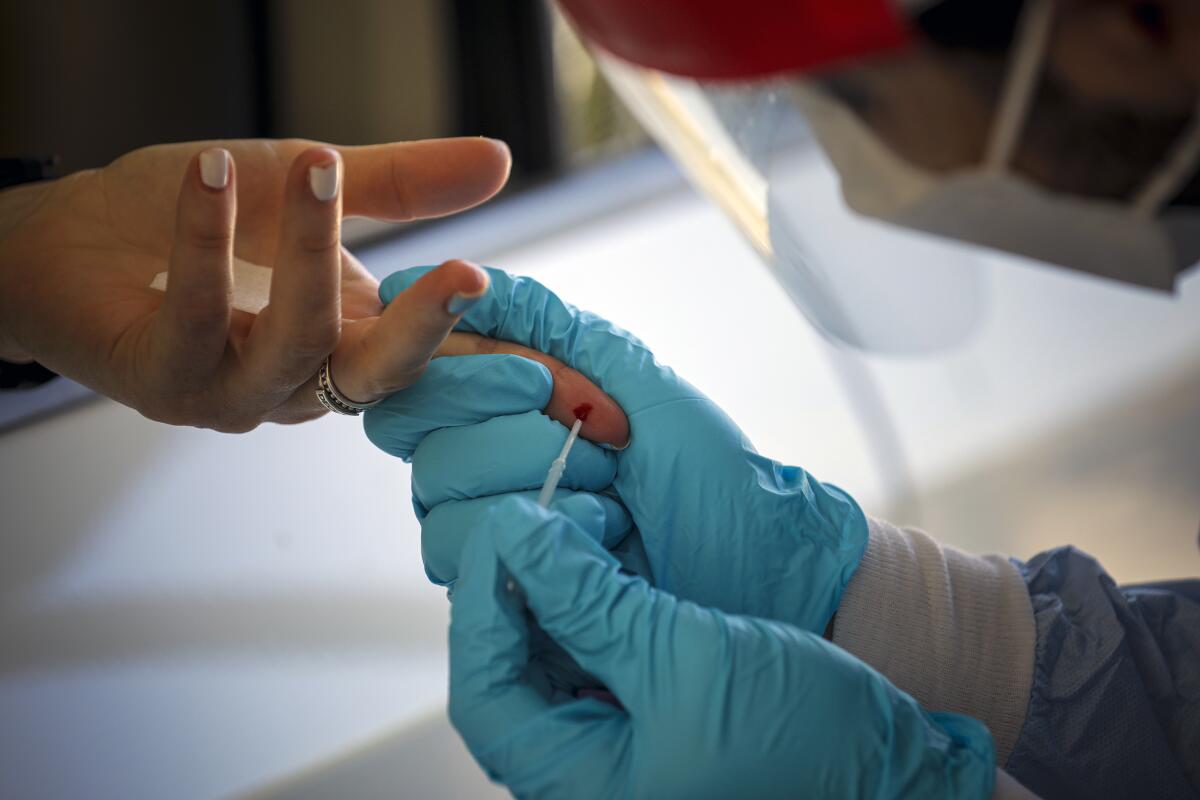
Antibody tests are different than the nasal or oral swab exams that diagnose an active COVID-19 infection. Also known as serology tests, antibody tests signal whether a patient has been exposed to the virus in the past. Sometimes patients sit for a traditional blood draw that fills a whole vial and is shipped to a lab. Other times patients have their finger pricked for a single drop that’s analyzed in about 10 minutes.
Antibody testing plays an important role in large studies because those can give a wide snapshot of the spread of the disease. But experts are skeptical about the tests being marketed to individuals.
The tests are advertised on everything from Instagram to roadside tents. Many of them are made in the U.S., but products from other countries have also entered the market, including some from China that have raised serious concerns over their quality.
At least one major pharmacy chain, Walgreens, is offering antibody tests, in partnership with LabCorp, that have received emergency authorization from the FDA. At smaller clinics, though, it can be difficult for the average consumer to find out who makes the test being offered and whether it’s authorized.
Alpha Hydration Services, which operates an IV therapy lounge in Woodland Hills, sells a rapid finger-stick blood test for $125. The venue specializes in hooking people up to IVs for “beauty drips” or “wellness drips” of vitamins but doesn’t normally provide blood testing.
Infectious disease experts are raising questions about the reliability of early coronavirus antibody tests and studies that hinge on their results.
The owner, Dwayne Mariner, a registered nurse, said a local vendor sold him the tests, which he believes were made in Asia and bear the name iCare. He said they have only about 70% accuracy. iCare is not among the manufacturers that have received emergency authorization from the FDA.
“Clinically, you could use this as a guideline for being out in public. Maybe you don’t need a mask while you’re walking around the neighborhood” if you test positive for antibodies, he said.
Mariner said he does not advise his patients on how to interpret the results and acknowledged the tests haven’t been rigorously inspected. Still, he suggested that patients who test positive for antibodies could use the information to determine for themselves whether to get a COVID-19 vaccine, once one is available.
“It gives someone more peace of mind if they didn’t want to get the vaccine anyway,” he said.
His comments are disputed by experts. It’s likely that people with positive antibody tests will still need the vaccine because scientists have not established that antibodies confer any immunity to COVID-19.
At the HOT Clinic, an IV therapy center in Encino, operations manager Edward Zaghikian said previously symptomatic patients who test negative for an active infection and either negative or positive for the presence of antibodies, then get tested again with the same results, can present the information to an employer showing they’re safe to return to work.
“You could take a calculated risk that it could be OK to come back to work,” said Zaghikian, whose company has administered about 1,500 of its $95 rapid antibody tests. He reasoned that a positive antibody test would mean the person has some immunity, and a negative result would mean they were never exposed and are thus virus-free.
But infectious disease experts warn against this logic. Just because someone had not previously been exposed to the virus doesn’t mean they won’t be in the future. The antibody tests shouldn’t be used to make any life decisions, they say.
“I think it is unwise for places to sell [the tests] because we do not know what the results of the test mean or what to do with that information,” said Davey Smith, a professor of medicine and head of UC San Diego’s Division of Infectious Diseases and Global Public Health.
Danielle Ompad, a professor of epidemiology at New York University, said the setting where people get tested matters, including whether healthcare professionals are on hand to explain what the test results mean for their patients going forward.
“If your chiropractor doesn’t normally do diagnostic testing, you shouldn’t be getting these tests done there,” she said.
And because there are potential flaws in this first generation of antibody tests, she said, people should stick to those that have received emergency authorization from the FDA.
Few tests being marketed — including ones from established labs — have such authorization.
A $249 antibody test at Next Health, a longevity center in Los Angeles, hasn’t been cleared by the FDA. The test’s manufacturer, Vibrant America, a certified lab, validated the test internally and has an application for emergency authorization pending before the federal agency.
Darshan Shah, founder and chief executive of Next Health, says he’s offering the test because he wants to give patients as much information as possible about their health during the pandemic. Shah, a physician, acknowledged the tests don’t have huge clinical significance in signaling how patients should be treated going forward or whether they can take fewer precautions, but his clinic is working to facilitate possible plasma donation by those who test positive for antibodies.

A rapid blood test made by Phamatech, available at the Westside Walk-in Clinic in Venice, which focuses on botox and coolsculpting, also doesn’t have emergency authorization from the FDA. Hannah Veal, the clinic’s owner, said she felt confident in selling it because the manufacturer has a good track record in rapid tests and has also submitted its validation data to the FDA, awaiting authorization.
Ideally, Veal said, she would only offer a test that has received emergency authorization.
“The problem is we are not in an ideal world right now. The reality is if manufacturers are making a test with 95% accuracy, why not offer it?” said Veal, a physician’s assistant, adding that she is open with her customers about the fact that the test is not FDA-approved.
Veal said some of her patients who test negative for the antibodies are disappointed that they have to continue wearing a mask. She said she counsels clients they shouldn’t get rid of their masks no matter their results.
Results from antibody tests could be sent to the federal Centers for Disease Control and Prevention, but it’s unclear whether the agency is receiving results from all possible test administrators.
There’s been talk of creating immunity passports for workers using coronavirus antibody tests, but they’re in short supply and not 100% accurate.
Experts say the tests may not even be worth getting for individual patients, other than to satisfy their curiosity.
“From a clinician’s perspective, [the testing] probably is not very useful right now,” said Flanigan, of the laboratory science group.
“I cannot think of any circumstances under which people in massage parlors or float tank studios should be giving people antibody tests,” he said.
Times researcher Scott Wilson and Times staff writer Sam Dean contributed to this report.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.