Column: A federal appeals court hands the FDA a stupendous win in its battle against stem cell clinics
Just as it began to seem that the battle against pseudoscience in medicine was being lost, a federal appeals court in San Francisco delivered a huge win for the Food and Drug Administration on its science-based regulation of stem cell clinics.
The three-member panel of the 9th Circuit Court of Appeals found that the FDA’s definition of processed stem cells as a “drug” was correct. The judges also accepted the FDA’s definition of its authority to regulate clinics offering purported stem cell treatments.
“The FDA’s interpretation... is the only interpretation that makes sense,” wrote Judge Jennifer Sung in her portion of a two-part unanimous ruling announced Friday.
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.
The ruling is important for the FDA’s campaign against medical clinics offering unproven treatments using patients’ stem cells.
Hundreds of such clinics have sprung up around the country, luring customers with claims that their treatments can address conditions including Alzheimer’s, arthritis, cancer, macular degeneration, Crohn’s disease, Parkinson’s and even erectile dysfunction. The FDA says no scientifically validated evidence supports any of those claims, and warns the public against stem cell-related “unscrupulous hype.”
This case was aimed at clinics operating under the labels Cell Surgical Network and California Stem Cell Treatment Center, which the FDA said were giving patients illegal drugs at clinics in Beverly Hills and Rancho Mirage.
A federal judge finds that a California stem cell clinic wasn’t violating FDA drug rules.
The agency had earlier won a case against a Florida stem cell clinic on claims that were virtually identical to those of the California case; that ruling in federal trial court was upheld by the 11th Circuit Court of Appeals in 2021.
The defendants also included Drs. Mark Berman and Elliott Lander, the enterprises’ founders and operators. Berman died in May 2022, a few months before federal Judge Jesus Bernal of Riverside ruled against the FDA in the case.
The appeals court overturned Bernal’s verdict and sent the case back to him for further proceedings. Lander and the clinics didn’t respond to my requests for comment on the appellate ruling.
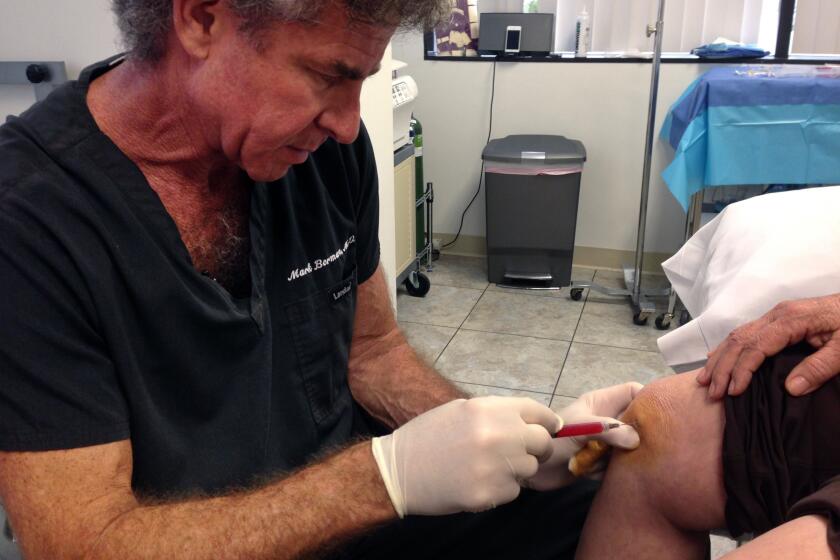
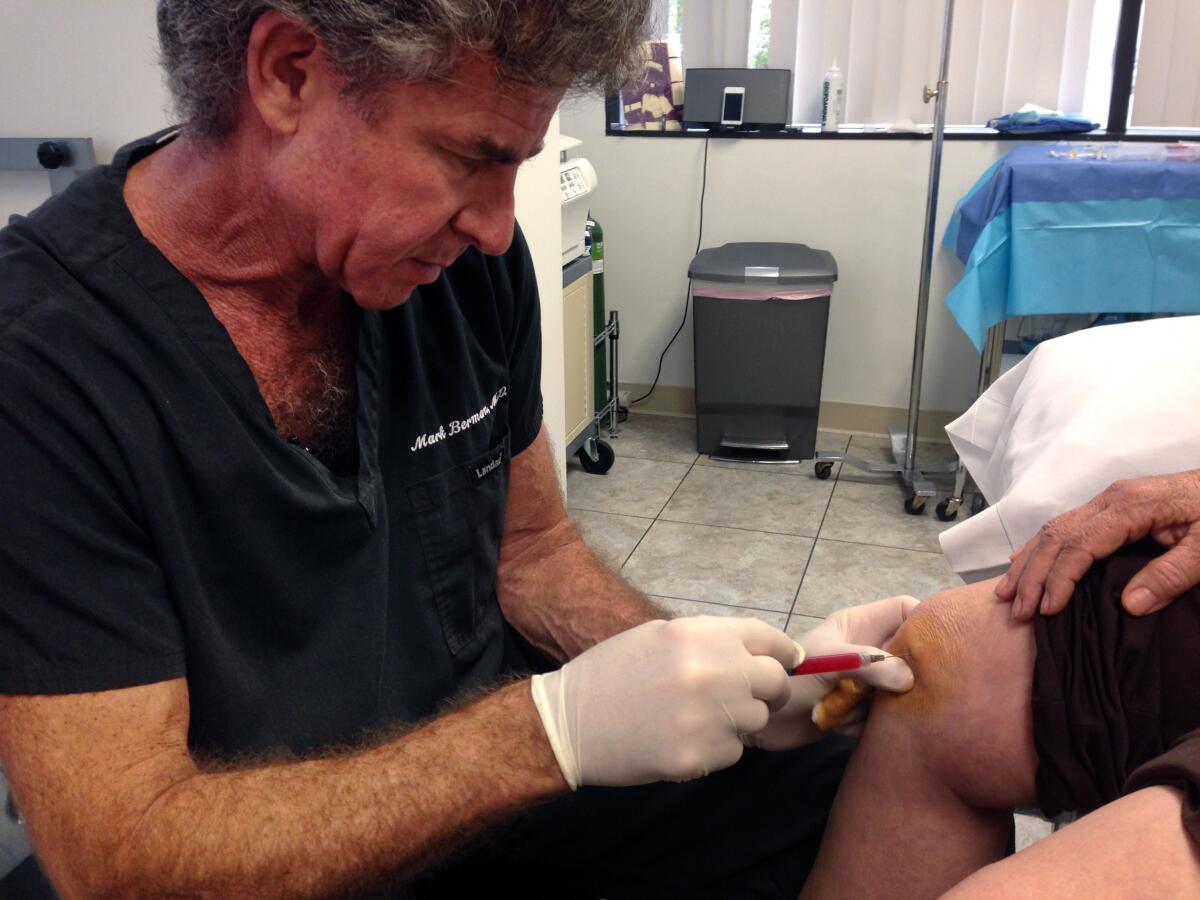
The clinics operated by extracting a patient’s fat by liposuction, processing the fat to extract stem cells and injecting the stem cells back into the patients. They typically charged $8,900 per treatment, or a package of 12 treatments for $41,500, the judges observed.
The defendants argued that their procedures fell within exemptions from FDA regulations. The key exemption applies to cells or tissues extracted and reimplanted in patients in a single surgical procedure. That exemption, the FDA argued, applies only when the cells or tissues aren’t changed or manipulated between extraction and reimplantation.
In sum, the FDA argued that what the clinics reimplanted was no longer identical to the fat, and also that process was more than a single surgical procedure. The appeals court ruled in the FDA’s favor on both points.
“This is a big victory for the FDA, patients, and the stem cell field,” said Paul Knoepfler, a biologist at UC Davis who has been tracking the proliferation of clinics selling unproven stem cell therapies for years. “It also fits nicely with [the] earlier ruling in the FDA’s favor...Together, these rulings seem coherent and establish that the FDA has the clear authority to regulate this class of unproven fat cell therapies as a drug.”
More to Read
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.