Column: Can a COVID-19 vaccine be tested without violating test subjects’ human rights?

You can think of Ian Haydon as a pioneer, or even as a heroic explorer into the unknown.
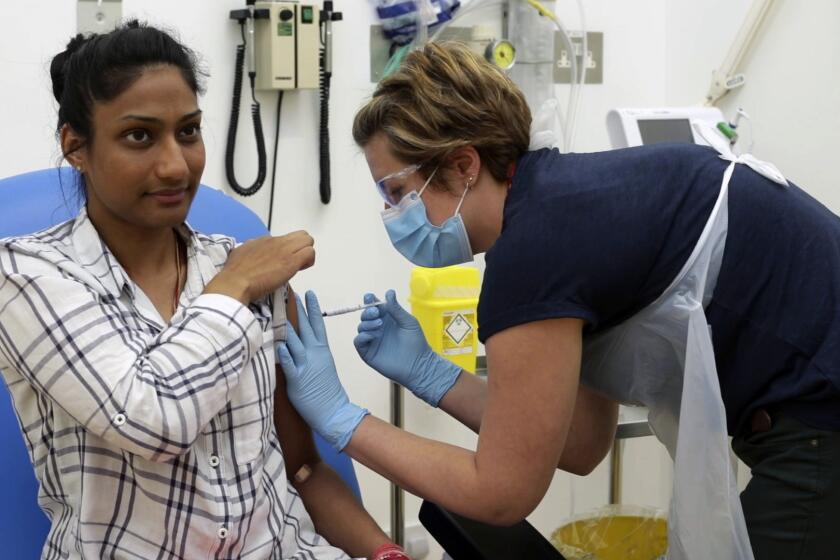
The 29-year-old press officer at the University of Washington volunteered earlier this year to become a test subject for an experimental COVID-19 vaccine being developed by Moderna Inc.
The aim of the so-called Phase 1 trial was to determine whether there were short-term adverse effects of the vaccine, and if so, how severe they were.
In order to develop a coronavirus vaccine, healthy people are going to need to step up and take a little bit of risk in these clinical trials.
— Vaccine trial volunteer Ian Haydon
For Haydon, they were pretty severe. After receiving his second shot of a high-dose version of the vaccine, he spiked a fever of more than 103 degrees, which led him to seek treatment at a local urgent care clinic. When he returned home, he lost consciousness.
A day later, however, the symptoms had disappeared, and Moderna touted Haydon’s experience (anonymized), as an indication that the vaccine had passed its initial trial threshold for safety.
Haydon told me he has had “no regrets whatsoever” about having participated in the trial. He has described himself as not “a big risk-taker.”
He didn’t sign up for the trial out of bravado or for pay, though he receives a modest monthly payment over the 14 months that his health will be monitored. He says he would participate in the trial even if it were unpaid.
“It’s a way I could responsibly use my health to help other people,” he says. “In order to develop a coronavirus vaccine, healthy people are going to need to step up and take a little bit of risk in these clinical trials. If people are unwilling to do that because they deem the risks to be too high, then we are just not going to get a vaccine.”
As clinical trials go, Haydon’s risk was relatively mild. He wasn’t exposed to the coronavirus (Moderna’s vaccine doesn’t use either a live or neutralized version of the bug), and although the vaccine was found to have given him antibodies that could fight the virus, he has subsequently tested negative for the virus itself.
A century ago, Sinclair Lewis predicted how America would botch the coronavirus crisis.
But as the numerous vaccine candidates being developed around the world move into more advanced trial phases, more questions are being asked about the ethics of the development programs.
In part that’s because of the need to speedily develop a vaccine for a virus that is unusually infectious and dangerous, causing an estimated 620,000 deaths worldwide in a matter of months and wreaking global economic havoc.
“Developing ... and distributing an efficacious vaccine as quickly as possible is a moral imperative for the world,” pediatrician Stanley Plotkin of the University of Pennsylvania and bioethicist Arthur Caplan of New York University wrote recently in the medical journal Vaccine.
The entire field of drug and vaccine testing has been fraught with ethical questions. The greatest scrutiny has been devoted to Phase 1 trials, around which an entire outsourcing industry has sprung to take the chore of preliminary testing off the hands of pharmaceutical companies.
As sociologist Jill Fisher documents in her recent book “Adverse Events,” Phase 1 trials, which once exploited mainly prisoners, now rely on a population of largely low-income subjects who enroll serially in drug trials, often confined in dreary facilities for the duration of the trials.
History tells us that rushing a vaccine for COVID-19 will prove more hazardous than if it’s done right.
Thanks to the public’s concern with the quick development and deployment of a COVID-19 vaccine, “there’s a lot more public attention on what it takes to get a vaccine on the market,” Fisher says. But much of that attention is devoted to ways to speed up the process, she says. We should be asking “what kind of corners we might be cutting to get something out there quicker.”
The most crucial ethical questions concern what are known as “challenge trials,” in which volunteers are deliberately exposed to a pathogen so the efficacy of a treatment can be determined more quickly than in the wild.
Instead of waiting for a pathogen to take root — or not — in a large population living in natural conditions, a challenge trial can deliver results faster and with fewer subjects.
“While we’re waiting, we’re seeing tremendous death rates and damage around the world, and that’s a pretty big price to pay for giving up months and years of speed,” Caplan told me.
Challenge trials are not novel; they’ve been used in research into treatments for influenza and other nonfatal diseases. The World Health Organization, in its ethical guidelines for challenge trials, mentions smallpox, yellow fever, typhoid and cholera.
The WHO also acknowledges, however, that “research involving the deliberate infection of healthy volunteers may seem intuitively unethical, and there are numerous prominent historical examples of unethical research involving deliberate infection of research subjects.”
America has fallen so far behind in the coronavirus fight that it might lose the war permanently.
Among the more notorious cases of unethical research is the Tuskegee syphilis study, in which penicillin was withheld from hundreds of Black men in Alabama known to have contracted syphilis even after it became known as an effective treatment for the disease.
Stephen Thomas, chief of the infectious diseases division at New York’s Upstate Medical University, wrote recently of having volunteered for a trial involving a potential vaccine for malaria in 2001.
“I figured if I was going to ask others to volunteer for my research experiments, I should be willing to do the same,” he wrote. He got the shot, was infected with malaria, and didn’t catch the disease. The test vaccine worked and has continued to undergo field testing.
The potential of using challenge trials to shorten the path to a safe and effective COVID-19 vaccine has been widely touted in the professional literature recently.
As Carl Elliott of the University of Minnesota has noticed, some of the drumbeat has come, interestingly enough from bioethicists trying to balance the considerable risks of the challenge protocol with the obvious social risks of a globally virulent disease.
“Every week that vaccine rollout is delayed will be accompanied by many thousands of deaths globally,” wrote Nir Eyal of Rutgers, Marc Lipsitch of Harvard and British epidemiologist Peter G. Smith in March. Challenge studies, they wrote, can be designed to “heed both individual rights and the global public health emergency.”
Others have gone further. Bioethicists Julian Savulescu and Dominic Wilkinson raised the possibility of asking “competent” nursing home residents to volunteer as expressions of “extreme altruism,” though they acknowledged that some of the volunteers “may be fatalistic or wish to die, or at any rate not care if they die sooner rather than later.”
Compared to previous challenge trials, deliberately infecting subjects with the coronavirus that causes COVID-19 raises the stakes considerably. For one thing, there’s no known cure for the disease, and not even many treatments known to have a significant effect on its progress.
“Assuming that we can infect someone, is there a reasonable treatment strategy that can rescue that person if they start to have more severe disease than we expect or what we want?” Thomas asks.
The stock market is euphoric over news of a possible coronavirus vaccine. But for now, you shouldn’t be.
Despite encouraging trial results for several treatment options, he says, “Right now, the medical field would probably not say that there is a rescue therapy that everyone would have 100% confidence in, like we have for malaria.”
Thomas, Caplan and others say that COVID vaccine challenge trials should be painstakingly designed to protect volunteers.
“The first thing we should ask is why we need to be doing a challenge trial instead of proceeding with the normal development pathway,” Thomas says. “Then we should ask whether the risk to the individual is reasonable. You would never do a challenge trial with Ebola or HIV.”
Caplan agrees that challenge trials might not be absolutely necessary, but argues that “we should have it in our arsenal as an option.” If the vaccine candidates now under study fall short of expected results, the ability to enroll subjects for trials of subsequent candidates may shrink, making challenge trials more necessary.
“You want to have challenge trials in your pocket if none of the first 10 vaccines work,” Caplan says.
Steps would have to be taken to ensure that volunteers “fully comprehend the unusual risks involved in the study,” Eyal, Lipsitch and Smith wrote.
Yet whether anyone can adequately understand the risks is questionable. A large number of volunteers would receive a placebo rather than a test vaccine, meaning that they could be exposed to the infection without even experimental immunization.
The long-term consequences of COVID-19 infection are not yet understood; some indications exist that the virus wreaks such havoc on several biological systems that patients could suffer some effects for a lifetime.
A glitch in the Social Security formula means a longterm coronavirus-related benefit cut for some workers
Advocates of challenge testing say that volunteers must be guaranteed lifelong treatment for the consequences, but it may not be a simple matter to link a health problem occurring years or even decades from now with an infection experienced today.
Challenge trials could be designed to enroll subjects whose risk of complications or death is statistically low — young adults, for instance — and who are naturally exposed to the virus, such as healthcare workers or residents of virus hot spots.
(The WHO recommends subjects ages 18-30, and cautions against enrolling subjects who are already socially disadvantaged, such as the poor.)
Volunteers would have the advantage of close medical observation and access to the best treatments known if they do become infected.
At the moment, public interest and enthusiasm for challenge trials appears to be sizable. The advocacy organization 1Day Sooner says it has signed up more than 32,000 volunteers from 140 countries, despite the manifest risks.
The impetus to proceed with extraordinary efforts remains strong as the coronavirus continues to spread globally. Plotkin and Caplan closed their paper by citing a line from “Hamlet” pointing to the urgency of taking every possible step to eradicate COVID-19: “Diseases desperate grown by desperate appliance are relieved, or not at all.”
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.